Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis - PubMed (original) (raw)
Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis
Christian Braendle et al. PLoS Biol. 2003 Oct.
Abstract
Symbiotic relationships between bacteria and insect hosts are common. Although the bacterial endosymbionts have been subjected to intense investigation, little is known of the host cells in which they reside, the bacteriocytes. We have studied the development and evolution of aphid bacteriocytes, the host cells that contain the endosymbiotic bacteria Buchnera aphidicola. We show that bacteriocytes of Acyrthosiphon pisum express several gene products (or their paralogues): Distal-less, Ultrabithorax/Abdominal-A, and Engrailed. Using these markers, we find that a subpopulation of the bacteriocytes is specified prior to the transmission of maternal bacteria to the embryo. In addition, we discovered that a second population of cells is recruited to the bacteriocyte fate later in development. We experimentally demonstrate that bacteriocyte induction and proliferation occur independently of B. aphidicola. Major features of bacteriocyte development, including the two-step recruitment of bacteriocytes, have been conserved in aphids for 80-150 million years. Furthermore, we have investigated two cases of evolutionary loss of bacterial symbionts: in one case, where novel extracellular, eukaryotic symbionts replaced the bacteria, the bacteriocyte is maintained; in another case, where symbionts are absent, the bacteriocytes are initiated but not maintained. The bacteriocyte represents an evolutionarily novel cell fate, which is developmentally determined independently of the bacteria. Three of five transcription factors we examined show novel expression patterns in bacteriocytes, suggesting that bacteriocytes may have evolved to express many additional transcription factors. The evolutionary transition to a symbiosis in which bacteria and an aphid cell form a functional unit, similar to the origin of plastids, has apparently involved extensive molecular adaptations on the part of the host cell.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
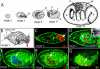
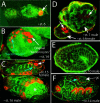
Figure 1. Expression of Three Transcription Factors during Early Bacteriocyte Development
(A) Drawings of some stages of pea aphid embryonic development, approximately to scale. Embryos develop viviparously within a follicular epithelium of the ovariole (data not shown). For a complete description, see Miura et al. (2003). Bacteria are transferred at stage 7. Embryos are labeled with bacteria (b), head (h), thoracic (t), and abdominal (a) regions. The three thoracic segments (t1, t2, t2) and germ cells (gc) are indicated in the stage 14 embryo. (B) A drawing of a stage 7 embryo illustrates transovarial transfer of the bacteria (red arrowhead) to the embryo and the presumptive bacteriocyte nuclei (arrow). (C) Confocal micrograph of a stage 6 embryo stained with anti-Dll antibody (red, indicated by arrow). Anti-Dll labels syncytial nuclei (presumptive bacteriocyte nuclei) in the posterior of the embryo. (D) Confocal micrograph of stage 7 embryo stained with anti-Dll and FP6.87 antibodies. Soon after the bacteria begin to invade the embryo, we observe staining with the FP6.87 antibody localized to the nucleoli (blue), which recognizes both Ubx and Abd-A in diverse arthropods, in the same nuclei that are already expressing Dll (red). The region outlined with a broken white box is enlarged in (D′) to show the bacteria, and only the green channel is shown in monochrome. The red arrow indicates one bacterium. (E and F) In these two panels of the same focal plane from the same stage 9 embryo, Ubx/Abd-A staining (blue) is observed throughout the entire nucleus of all nuclei that also express Dll (red). (G) Confocal micrograph of a stage 8 embryo stained with anti-En (yellow). As the transfer of bacteria (arrowhead) is being completed, the bacteriocyte nuclei begin to express En (yellow, indicated with arrow). In (C)–(G), confocal micrographs show only one focal plane of the embryo, so not all bacteriocyte nuclei in each embryo can be seen. In all figures, F-actin is stained with phalloidin (green). Embryos in all figures, except Figure 2, are oriented with anterior of the entire embryo (towards the germarium) to the left.
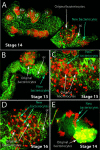
Figure 2. The Second Wave of Bacteriocyte Determination
In (A)–(D), the embryos, which are normally folded in upon themselves in a pretzel shape within the ovariole (Miura et al. 2003), have been dissected flat, stained with anti-Dll antibody (red) and phalloidin (green), and examined with a confocal microscope. (A) Dll expression (red) in a stage 14 embryo is detected in the labrum (La) and all developing limbs on the ventral surface except the mandibular segment (Mn). (Other abbreviations: An, antenna; Mx, maxilla; Lb, labium; T1, T2, T3, first, second, and third thoracic leg, respectively.) The dorsal surface of the abdomen of the same embryo is shown illustrating Dll expression in the original bacteriocytes (white arrow) and in a more posterior population of nuclei or cells (blue arrow). Germ cells (gc) are labeled. (B) Dll expression is first observed in the new bacteriocyte nuclei at stage 13. (C) By stage 15, many of the new bacteriocytes have migrated to and begun intercalating between the original bacteriocytes. (D) By stage 16, all of the new bacteriocytes have intercalated between the original bacteriocytes. (E) The migration of the new bacteriocytes is seen in a confocal section of an undissected stage 14 embryo. Embryos in (A)–(D) are oriented with the anterior of the germband towards the left.
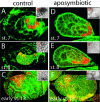
Figure 3. Elimination of B. aphidicola by Treatment with Antibiotics Has No Effect on the Determination and Maintenance of the Bacteriocyte Cell Fate in A. pisum
(A–C) Confocal micrographs of control embryos stained with anti-Dll antibody (red) show expression of Dll, as described in Figure 1. Enlarged views of the bacteria within the broken white boxes in each embryo are shown in (A′)–(C′). (D–F) Embryos within aposymbiotic aphids at comparable stages as the controls in (A)–(C) express Dll in bacteriocyte nuclei. No bacteria are observed within these embryos, as seen in the enlarged views of (D′)–(F′).
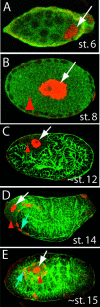
Figure 4. Expression of Dll in Bacteriocytes and the Pattern of Bacteriocyte Development Are Conserved in Parthenogenetic Females of P. spyrothecae
Confocal micrographs of P. spyrothecae parthenogenetic embryos stained with anti-Dll antibody (red). (A) Dll is first detected in stage 6 embryos in one or two nuclei posterior to the cellular blastoderm (arrow). (B) By stage 8, the bacteria have been transferred to and entirely fill the embryo (red arrowhead). The Dll-expressing nuclei (arrow) have become highly polyploid. (C and D) At stage 12, only the original bacteriocyte nuclei are observed expressing Dll (white arrow), but by stage 14 (D) additional nuclei (blue arrow) closely apposed to the dorsal germband express Dll. (E) By stage 15, these new nuclei surround the original bacteriocyte, and at later stages the bacteria are divided into individual cells.
Figure 5. Bacteriocytes Are Retained in One Species That Has Evolutionarily Lost Bacteria, but Not in Males of Another Species That Do Not Inherit Bacteria
(A and B) Confocal micrographs of embryos of T. styraci stained with anti-Dll antibody (red). In T. styraci, in which B. aphidicola has been evolutionarily lost (Fukatsu and Ishikawa 1992a), embryos still contain nuclei that express Dll in the correct time and place to be bacteriocyte nuclei. (A) Dll expression is first detected in posterior nuclei at blastoderm at approximately stage 6 (arrow). (B) By stage 14, the original nuclei have divided once or twice and become polyploid (original bacteriocytes), and new cells begin to express Dll (new bacteriocytes; blue arrow) and migrate towards the original bacteriocytes. (C–F) Confocal micrographs of embryos of P. spyrothecae stained with anti-Dll antibody (red). (C) Stage 16 male embryos of P. spyrothecae do not contain B. aphidicola, and no Dll-expressing cells are observed in the expected location for bacteriocytes. We believe that the cells in this location are sperm (marked with an asterisk). Sexual female embryos within the same ovary do contain Dll-expressing original and new bacteriocyte nuclei (white and blue arrows, respectively). (D and E) Transient expression of Dll in putative bacteriocytes is observed in stage 7 male embryos (arrow in male embryo of [D]), but this expression does not persist into stage 10 male embryos (E), where no Dll-expressing nuclei are observed. By contrast, stage 6 female embryos (D) contain polyploid Dll-expressing nuclei (arrow in female embryo of [D]). The sex of each embryo could be determined because males develop synchronously and earlier than females (Lampel 1958, 1968). (F) In stage 14 male embryos, we observe transient Dll expression in nuclei (blue arrow) adjacent to the germ cells (gc) in the correct location to be the second wave of bacteriocyte nuclei. This Dll expression does not persist (see stage 16 male in [C]), and the fate of the cells is unknown.
Similar articles
- Genome expansion and differential expression of amino acid transporters at the aphid/Buchnera symbiotic interface.
Price DR, Duncan RP, Shigenobu S, Wilson AC. Price DR, et al. Mol Biol Evol. 2011 Nov;28(11):3113-26. doi: 10.1093/molbev/msr140. Epub 2011 May 24. Mol Biol Evol. 2011. PMID: 21613235 - A dual-genome microarray for the pea aphid, Acyrthosiphon pisum, and its obligate bacterial symbiont, Buchnera aphidicola.
Wilson AC, Dunbar HE, Davis GK, Hunter WB, Stern DL, Moran NA. Wilson AC, et al. BMC Genomics. 2006 Mar 14;7:50. doi: 10.1186/1471-2164-7-50. BMC Genomics. 2006. PMID: 16536873 Free PMC article. - Aphid amino acid transporter regulates glutamine supply to intracellular bacterial symbionts.
Price DR, Feng H, Baker JD, Bavan S, Luetje CW, Wilson AC. Price DR, et al. Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):320-5. doi: 10.1073/pnas.1306068111. Epub 2013 Dec 23. Proc Natl Acad Sci U S A. 2014. PMID: 24367072 Free PMC article. - Insect Bacteriocytes: Adaptation, Development, and Evolution.
Luan JB. Luan JB. Annu Rev Entomol. 2024 Jan 25;69:81-98. doi: 10.1146/annurev-ento-010323-124159. Annu Rev Entomol. 2024. PMID: 38270981 Review. - Genetics, physiology, and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids.
Baumann P, Baumann L, Lai CY, Rouhbakhsh D, Moran NA, Clark MA. Baumann P, et al. Annu Rev Microbiol. 1995;49:55-94. doi: 10.1146/annurev.mi.49.100195.000415. Annu Rev Microbiol. 1995. PMID: 8561471 Review.
Cited by
- Complex host/symbiont integration of a multi-partner symbiotic system in the eusocial aphid Ceratovacuna japonica.
Yorimoto S, Hattori M, Kondo M, Shigenobu S. Yorimoto S, et al. iScience. 2022 Nov 2;25(12):105478. doi: 10.1016/j.isci.2022.105478. eCollection 2022 Dec 22. iScience. 2022. PMID: 36404929 Free PMC article. - Identification of the weevil immune genes and their expression in the bacteriome tissue.
Anselme C, Pérez-Brocal V, Vallier A, Vincent-Monegat C, Charif D, Latorre A, Moya A, Heddi A. Anselme C, et al. BMC Biol. 2008 Oct 16;6:43. doi: 10.1186/1741-7007-6-43. BMC Biol. 2008. PMID: 18925938 Free PMC article. - Sugar, amino acid and inorganic ion profiling of the honeydew from different hemipteran species feeding on Abies alba and Picea abies.
Shaaban B, Seeburger V, Schroeder A, Lohaus G. Shaaban B, et al. PLoS One. 2020 Jan 24;15(1):e0228171. doi: 10.1371/journal.pone.0228171. eCollection 2020. PLoS One. 2020. PMID: 31978201 Free PMC article. - Bacteriocyte cell death in the pea aphid/Buchnera symbiotic system.
Simonet P, Gaget K, Balmand S, Ribeiro Lopes M, Parisot N, Buhler K, Duport G, Vulsteke V, Febvay G, Heddi A, Charles H, Callaerts P, Calevro F. Simonet P, et al. Proc Natl Acad Sci U S A. 2018 Feb 20;115(8):E1819-E1828. doi: 10.1073/pnas.1720237115. Epub 2018 Feb 5. Proc Natl Acad Sci U S A. 2018. PMID: 29432146 Free PMC article. - The molecular basis of bacterial-insect symbiosis.
Douglas AE. Douglas AE. J Mol Biol. 2014 Nov 25;426(23):3830-7. doi: 10.1016/j.jmb.2014.04.005. Epub 2014 Apr 13. J Mol Biol. 2014. PMID: 24735869 Free PMC article. Review.
References
- Baumann P, Moran NA, Baumann L, editors. Bacteriocyte-associated endosymbionts of insects. In: Dworkin M, editor. The prokaryotes [online] New York: Springer; 2000. Available: http://link.springer.de/link/service/books/10125/
- Buchner P, editor. Endosymbiosis of animals with plant microorganisms. New York: John Wiley; 1965. 909 pp.
- Caillaud CM, Rahbé Y. Aposymbiosis in a cereal aphid: Reproductive failure and influence on plant utilization. Ecol Entomol. 1999;24:111–114.
- Chang CC, Dearden P, Akam M. Germ line development in the grasshopper Schistocerca gregaria: Vasa as a marker. Dev Biol. 2002;252:100–118. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous