Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection - PubMed (original) (raw)
Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection
Stephen P Saville et al. Eukaryot Cell. 2003 Oct.
Abstract
It is widely assumed that the ability of Candida albicans to switch between different morphologies is required for pathogenesis. However, most virulence studies have used mutants that are permanently locked into either the yeast or filamentous forms which are avirulent but unsuitable for discerning the role of morphogenetic conversions at the various stages of the infectious process. We have constructed a strain in which this developmental transition can be externally modulated both in vitro and in vivo. This was achieved by placing one copy of the NRG1 gene (a negative regulator of filamentation) under the control of a tetracycline-regulatable promoter. This modified strain was then tested in an animal model of hematogenously disseminated candidiasis. Mice injected with this strain under conditions permitting hyphal development succumbed to the infection, whereas all of the animals injected under conditions that inhibited this transition survived. Importantly, fungal burdens were almost identical in both sets of animals, indicating that, whereas filament formation appears to be required for the mortality resulting from a deep-seated infection, yeast cells play an important role early in the infectious process by extravasating and disseminating to the target organs. Moreover, these infecting Candida yeast cells still retained their pathogenic potential, as demonstrated by allowing this developmental transition to occur at various time points postinfection. We demonstrate here the importance of morphogenetic conversions in C. albicans pathogenesis. This engineered strain should provide a useful tool in unraveling the individual contributions of the yeast and filamentous forms at various stages of the infectious process.
Figures
FIG. 1.
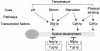
Schematic representation of pathways leading to hyphal development in C. albicans. In contrast to the transcription factors Efg1p and Cph1p, whose activation is required for hyphal development, Nrg1p functions as a negative regulator, preventing the transcription of hyphal specific genes in yeast form cells. Nrg1p is a DNA-binding protein that associates with elements upstream of hyphal specific genes and forms a complex with the general repressor protein Tup1p. In contrast to TUP1, which is constitutively transcribed,NRG1 expression is downregulated after appropriate stimuli, thereby facilitating hypha-specific gene transcription (adapted from reference 7).
FIG. 2.
Construction of strain SSY50-B. (A) Diagram depicting the construction of the NRG1 promoter replacing construct and its introduction into strain THE1. This yeast strain contains the TR transactivator gene required to drive expression from the tetO promoter sequence introduced upstream of NRG1. (B) Southern blot analysis confirming integration of the promoter replacing fragment at the NRG1 locus in strains SSY50-B, -F, and -H. Genomic DNA prepared from the three Ura+ transformants and the parental THE1 was digested with Sty_I or Xba_I and probed with the NRGB PCR product described in the text. Note that the DNA prepared from the Ura+ strains has an additional larger hybridizing fragment as a consequence of the integration of the_URA3 and tetO sequences into one copy of the_NRG1 gene (both alleles are the same size in the THE1 parental strain).
FIG. 3.
Northern blot analysis displaying the effect of doxycycline on the wild-type and modified NRG1 alleles in strain SSY50-B. Total RNA was prepared from the CAF2-1 (wild-type) and SSY50-B strains after growth for 5 h under various conditions and then hybridized with an_NRG1_ probe. The conditions under which the cells were grown are indicated above the blot; lanes labeled 37S are those supplemented with 10% fetal calf serum. Note that the modified strain contains two hybridizing bands: one identical in size and regulated like the band seen in the CAF2-1 wild-type strain and a second, smaller band whose expression is completely dependent on the presence or absence of doxycycline in the medium. Also note the elevated expression, with respect to the message seen in the wild-type strain, of the smaller band in the absence of doxycycline.
FIG. 4.
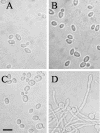
Microscopic evaluation of the effect of doxycycline on the morphogenetic transition in the SSY50-B modified strain. Samples were taken from cultures grown under various yeast- and hypha-inducing conditions for 5 h in the presence or absence of doxycycline, and their morphology was examined microscopically. (A) YPD without doxycycline at 37°C; (B) YPD plus serum without doxycycline at 37°C (cells fail to form filaments under inducing conditions); (C) YPD plus doxycycline at 25°C; (D) YPD plus doxycycline at 37°C (cells form filaments without the need for serum). Scale bar, 10 μm.
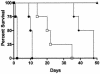
FIG. 5.
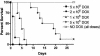
Control of NRG1 expression by doxycycline affects the outcome of haematogenously disseminated candidiasis caused by strain SSY50-B. Groups of mice either on 5% sucrose alone (NO DOX) or on sucrose containing doxycycline (DOX) were injected, as indicated, with different doses of the modified strain SSY50-B grown overnight in the absence of doxycycline, and their survival was monitored over a period of 28 days. While mice on doxycycline succumbed to the infection at rates similar to those seen with wild-type strains, every mouse not exposed to the antibiotic survived even at the highest dose tested (5 × 106 CFU). Statistically significant differences were observed between each two groups of mice injected with the same yeast inoculum in the presence or absence of doxycycline in their drinking water (P < 0.01 for all comparisons).
FIG. 6.
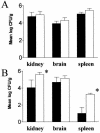
Fungal burden in the kidney, brain, and spleen of mice 6 h after infection with 5 × 106 cells (A) and 3 days after infection with 5 × 105 cells (B) of strain SSY50-B. Solid bars represent results obtained with mice on 5% sucrose containing 2 mg of doxycycline/ml; open bars represent results obtained with mice on sucrose only. Note the remarkable result that the fungal burdens are similar, irrespective of the animal's exposure to doxycycline, implying that the modified strain is able to efficiently extravasate while prevented from making the yeast-to-hypha transition. ✽, Statistically significant differences (P < 0.01).
FIG. 7.
Histopathological analysis of kidneys retrieved from mice infected with strain SSY50-B in the presence or absence of doxycycline. (A and B) Scattered yeast cells (A) and yeast microabscess (B) in the kidneys of antibiotic-free mice sacrificed 3 days after infection. (C and D) Hyphal elements (C) and extensive mycelial lesions (D) in kidneys recovered from doxycycline-treated mice succumbing to infection. Scale bars: 20 μm (A, B, and C) and 80 μm (D).
FIG. 8.
Delayed switch experiments to demonstrate that the yeast cells within the animal host are still capable of undergoing the morphogenetic transition. Groups of mice (n = 4) that were either pretreated (▪), not treated (▴), or switched to doxycycline at 3 (•), 8 (□), or 14 (♦) days after injection with 2 × 106 cells of strain SSY50-B were monitored for their ability to survive the infection. All comparisons between survival curves for each two groups resulted in statistically significant differences (P ≤ 0.01).
Similar articles
- Inhibition of filamentation can be used to treat disseminated candidiasis.
Saville SP, Lazzell AL, Bryant AP, Fretzen A, Monreal A, Solberg EO, Monteagudo C, Lopez-Ribot JL, Milne GT. Saville SP, et al. Antimicrob Agents Chemother. 2006 Oct;50(10):3312-6. doi: 10.1128/AAC.00628-06. Antimicrob Agents Chemother. 2006. PMID: 17005810 Free PMC article. - Examination of the pathogenic potential of Candida albicans filamentous cells in an animal model of haematogenously disseminated candidiasis.
Cleary IA, Reinhard SM, Lazzell AL, Monteagudo C, Thomas DP, Lopez-Ribot JL, Saville SP. Cleary IA, et al. FEMS Yeast Res. 2016 Mar;16(2):fow011. doi: 10.1093/femsyr/fow011. Epub 2016 Feb 5. FEMS Yeast Res. 2016. PMID: 26851404 Free PMC article. - Physiologic expression of the Candida albicans pescadillo homolog is required for virulence in a murine model of hematogenously disseminated candidiasis.
Uppuluri P, Chaturvedi AK, Jani N, Pukkila-Worley R, Monteagudo C, Mylonakis E, Köhler JR, Lopez Ribot JL. Uppuluri P, et al. Eukaryot Cell. 2012 Dec;11(12):1552-6. doi: 10.1128/EC.00171-12. Epub 2012 Oct 26. Eukaryot Cell. 2012. PMID: 23104566 Free PMC article. - The distinct morphogenic states of Candida albicans.
Sudbery P, Gow N, Berman J. Sudbery P, et al. Trends Microbiol. 2004 Jul;12(7):317-24. doi: 10.1016/j.tim.2004.05.008. Trends Microbiol. 2004. PMID: 15223059 Review. - Candida albicans dimorphism as a therapeutic target.
Jacobsen ID, Wilson D, Wächtler B, Brunke S, Naglik JR, Hube B. Jacobsen ID, et al. Expert Rev Anti Infect Ther. 2012 Jan;10(1):85-93. doi: 10.1586/eri.11.152. Expert Rev Anti Infect Ther. 2012. PMID: 22149617 Review.
Cited by
- Global analysis of fungal morphology exposes mechanisms of host cell escape.
O'Meara TR, Veri AO, Ketela T, Jiang B, Roemer T, Cowen LE. O'Meara TR, et al. Nat Commun. 2015 Mar 31;6:6741. doi: 10.1038/ncomms7741. Nat Commun. 2015. PMID: 25824284 Free PMC article. - Protection of Candida parapsilosis from neutrophil killing through internalization by human endothelial cells.
Glass KA, Longley SJ, Bliss JM, Shaw SK. Glass KA, et al. Virulence. 2015;6(5):504-14. doi: 10.1080/21505594.2015.1042643. Virulence. 2015. PMID: 26039751 Free PMC article. - Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans.
Navarathna DH, Hornby JM, Krishnan N, Parkhurst A, Duhamel GE, Nickerson KW. Navarathna DH, et al. Infect Immun. 2007 Apr;75(4):1609-18. doi: 10.1128/IAI.01182-06. Epub 2007 Feb 5. Infect Immun. 2007. PMID: 17283095 Free PMC article. - Limited role of secreted aspartyl proteinases Sap1 to Sap6 in Candida albicans virulence and host immune response in murine hematogenously disseminated candidiasis.
Correia A, Lermann U, Teixeira L, Cerca F, Botelho S, da Costa RM, Sampaio P, Gärtner F, Morschhäuser J, Vilanova M, Pais C. Correia A, et al. Infect Immun. 2010 Nov;78(11):4839-49. doi: 10.1128/IAI.00248-10. Epub 2010 Aug 2. Infect Immun. 2010. PMID: 20679440 Free PMC article. - Linking Cellular Morphogenesis with Antifungal Treatment and Susceptibility in Candida Pathogens.
Sharma J, Rosiana S, Razzaq I, Shapiro RS. Sharma J, et al. J Fungi (Basel). 2019 Feb 21;5(1):17. doi: 10.3390/jof5010017. J Fungi (Basel). 2019. PMID: 30795580 Free PMC article. Review.
References
- Berman, J., and P. E. Sudbery. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918-930. - PubMed
- Braun, B. R., and A. D. Johnson. 1997. Control of filament formation in Candida albicans by the transcriptional repressor, TUP1. Science 277:105-109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases