Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells - PubMed (original) (raw)
Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells
Noriyuki Ouchi et al. J Biol Chem. 2004.
Abstract
Adiponectin is an adipocyte-specific adipocytokine with anti-atherogenic and anti-diabetic properties. Here, we investigated whether adiponectin regulates angiogenic processes in vitro and in vivo. Adiponectin stimulated the differentiation of human umbilical vein endothelium cells (HUVECs) into capillary-like structures in vitro and functioned as a chemoattractant in migration assays. Adiponectin promoted the phosphorylation of AMP-activated protein kinase (AMPK), protein kinase Akt/protein kinase B, and endothelial nitric oxide synthesis (eNOS) in HUVECs. Transduction with either dominant-negative AMPK or dominant-negative Akt abolished adiponectin-induced eNOS phosphorylation as well as adiponectin-stimulated HUVEC migration and differentiation. Dominant-negative AMPK also inhibited adiponectin-induced Akt phosphorylation, suggesting that AMPK is upstream of Akt. Dominant-negative Akt or the phosphatidylinositol 3-kinase inhibitor LY294002 blocked adiponectin-stimulated Akt and eNOS phosphorylation, migration, and differentiation without altering AMPK phosphorylation. Finally, adiponectin stimulated blood vessel growth in vivo in mouse Matrigel plug implantation and rabbit corneal models of angiogenesis. These data indicate that adiponectin can function to stimulate the new blood vessel growth by promoting cross-talk between AMP-activated protein kinase and Akt signaling within endothelial cells.
Figures
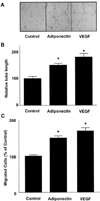
Fig. 1. Adiponectin promotes endothelial cell migration and differentiation into tube-like structures
Tube formation assays were performed (A and B). HUVECs were seeded on Matrigel-coated culture dishes in the presence of adiponectin (30 µg/ml), VEGF (20 ng/ml), or BSA (30 µg/ml) (Control). A, representative cultures are shown. B, quantitative analysis of tube formation. C, a modified Boyden chamber assay was performed using HUVECs. HUVECs were treated with adiponectin (30 µg/ml), VEGF (20 ng/ml), or BSA (30 µg/ml) (Control). Results are shown as mean ± S.E. Results are expressed relative to control. *, p < 0.01 versus control.
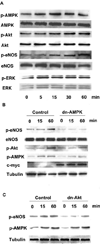
Fig. 2. Adiponectin-stimulated signaling in endothelial cells
A, time-dependent changes in the phosphorylation (_p_-) of AMPK, Akt, eNOS, and extracellular signal-regulated kinase (ERK) after adiponectin treatment (30 µg/ml). B, role of AMPK in the regulation of adiponectin-induced protein phosphorylation. HUVECs were transduced with an adenoviral vector expressing dominant-negative AMPK tagged with c-Myc (dn-AMPK) or an adenoviral vector expressing green fluorescence protein (Control) 24 h before serum-starvation. After 16-h serum starvation, cells were treated with adiponectin (30 µg/ml) for the indicated lengths of time. C, role of Akt in the regulation of adiponectin-induced protein phosphorylation. HUVECs were transduced with an adenoviral vector expressing dominant-negative Akt (dn-Akt) or an adenoviral vector expressing green fluorescence protein (Control) 24 h before serum starvation. After 16 h of serum starvation, cells were treated with adiponectin (30 µg/ml) for the indicated lengths of time. Representative blots are shown.
Fig. 3. Contribution of AMPK and Akt to adiponectin-induced angiogenic cellular responses
HUVECs were transduced with an adenoviral vector expressing dn-AMPK (gray), dn-Akt (open) or green fluorescence protein (Control, solid) 24 h before the change to low-serum media. After 16 h of serum starvation, in vitro Matrigel (A and B) or modified Boyden chamber assays (C) were performed. Cells were treated with adiponectin (30 µg/ml) or BSA (30 µg/ml) (Vehicle). A, representative cultures displaying tube formation are shown. B, quantitative analysis of tube lengths. C, modified Boyden chamber assay was performed with adiponectin or VEGF as chemoattractant. Results are shown as the mean ± S.E. Results are expressed relative to control. *, p < 0.01 versus each control.
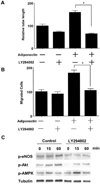
Fig. 4. PI3-kinase signaling is involved in adiponectin-induced angiogenic pathway
A, quantitative analysis of tube formation is shown. HUVECs were treated with adiponectin (30 µg/ml) or BSA (30 µg/ml) in the presence of LY294002 (10 µ
m
) or vehicle at the time of seeding. B, a modified Boyden chamber assay was performed using adiponectin as the chemoattractant. HUVECs were pretreated with LY294002 (10 µ
m
) or vehicle for 1 h and then incubated with adiponectin (30 µg/ml) or BSA (30 µg/ml) for 4 h. C, effects of LY294002 on adiponectin-stimulated protein phosphorylation (_p_-). Representative blots are shown. HUVECs were pretreated with LY294002 (10 µ
m
) or vehicle for 1 h and then incubated with adiponectin (30 µg/ml) or BSA (30 µg/ml) for the indicated lengths of time. Results are presented as the mean ± S.E. For A and B, results are expressed relative to control. *, p < 0.01.
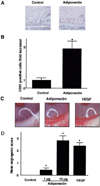
Fig. 5. Adiponectin promotes angiogenesis in vivo
An in vivo Matrigel plug assay was performed to evaluate the effect of adiponectin on angiogenesis (A and B). Matrigel plugs containing adiponectin (100 µg/ml, n = 3) or phosphate-buffered saline (Control, n = 3) were injected subcutaneously into mice. A, plugs were stained with the endothelial cell marker CD31. Bar, 100 µm. B, the frequency of CD31-positive cells in five low power fields was determined for each Matrigel plug. Data were presented as fold increase of CD31-positive cells relative to the control. A rabbit cornea assay was performed (C and D). Pellets containing adiponectin (1 and 10 µg, n = 8), VEGF (100 ng, n = 8), or phosphate-buffered saline (Control, n = 8) were implanted in the cornea. C, photographs of rabbit eyes are shown (Control, adiponectin 10 µg; VEGF, 100 ng). D, an angiogenic score was calculated (vessel density × distance from limbus). Results are shown as the mean ± S.E. *, p < 0.01 versus control.
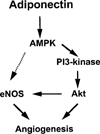
Fig. 6. Proposed scheme for adiponectin-stimulated signaling in endothelial cells
Adiponectin activates AMPK, which in turn promotes Akt activation, eNOS phosphorylation, and angiogenesis. PI3-kinase is essential for adiponectin-mediated activation of Akt. Both AMPK and Akt can directly phosphorylate eNOS. However, inhibition of Akt or PI3-kinase was found to suppress adiponectin-stimulated eNOS phosphorylation without interfering with AMPK activation. Therefore, the data are most consistent with an AMPK-PI3-kinase-Akt-eNOS-signaling axis.
Similar articles
- Adiponectin stimulates production of nitric oxide in vascular endothelial cells.
Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Chen H, et al. J Biol Chem. 2003 Nov 7;278(45):45021-6. doi: 10.1074/jbc.M307878200. Epub 2003 Aug 27. J Biol Chem. 2003. PMID: 12944390 - Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase.
Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Zou MH, et al. J Biol Chem. 2002 Sep 6;277(36):32552-7. doi: 10.1074/jbc.M204512200. Epub 2002 Jul 9. J Biol Chem. 2002. PMID: 12107173 - AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress.
Nagata D, Mogi M, Walsh K. Nagata D, et al. J Biol Chem. 2003 Aug 15;278(33):31000-6. doi: 10.1074/jbc.M300643200. Epub 2003 Jun 4. J Biol Chem. 2003. PMID: 12788940 - Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells.
Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Haynes MP, et al. Circ Res. 2000 Oct 13;87(8):677-82. doi: 10.1161/01.res.87.8.677. Circ Res. 2000. PMID: 11029403 - Nitric oxide and angiogenesis.
Ziche M, Morbidelli L. Ziche M, et al. J Neurooncol. 2000 Oct-Nov;50(1-2):139-48. doi: 10.1023/a:1006431309841. J Neurooncol. 2000. PMID: 11245273 Review.
Cited by
- Adiponectin-induced activation of ERK1/2 drives fibrosis in retinal pigment epithelial cells.
Palanisamy K, Karpagavalli M, Nareshkumar RN, Ramasubramanyan S, Angayarkanni N, Raman R, Chidambaram S. Palanisamy K, et al. Hum Cell. 2024 Oct 26;38(1):8. doi: 10.1007/s13577-024-01131-5. Hum Cell. 2024. PMID: 39460900 - Nanoetched Stainless Steel Architecture Enhances Cell Uptake of Biomacromolecules and Alters Protein Corona Abundancy.
Pho T, Janecka MA, Pustulka SM, Champion JA. Pho T, et al. ACS Appl Mater Interfaces. 2024 Oct 30;16(43):58427-58438. doi: 10.1021/acsami.4c14492. Epub 2024 Oct 17. ACS Appl Mater Interfaces. 2024. PMID: 39417567 Free PMC article. - Adiponectin Resistance in Obesity: Adiponectin Leptin/Insulin Interaction.
Engin A. Engin A. Adv Exp Med Biol. 2024;1460:431-462. doi: 10.1007/978-3-031-63657-8_15. Adv Exp Med Biol. 2024. PMID: 39287861 Review. - A Preliminary Study on Factors That Drive Patient Variability in Human Subcutaneous Adipose Tissues.
DeBari MK, Johnston EK, Scott JV, Ilzuka E, Sun W, Webster-Wood VA, Abbott RD. DeBari MK, et al. Cells. 2024 Jul 24;13(15):1240. doi: 10.3390/cells13151240. Cells. 2024. PMID: 39120271 Free PMC article. - The mysterious association between adiponectin and endometriosis.
Zhao YQ, Ren YF, Li BB, Wei C, Yu B. Zhao YQ, et al. Front Pharmacol. 2024 May 15;15:1396616. doi: 10.3389/fphar.2024.1396616. eCollection 2024. Front Pharmacol. 2024. PMID: 38813109 Free PMC article. Review.
References
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Nature. 1994;372:425–432. - PubMed
- Hotamisligil GS, Shargill NS, Spiegelman BM. Science. 1993;259:87–91. - PubMed
- Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y. Nat. Med. 1996;2:800–803. - PubMed
- Trayhurn P, Beattie JH. Proc. Nutr. Soc. 2001;60:329–339. - PubMed
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. Biochem. Biophys. Res. Commun. 1996;221:286–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD23681/HD/NICHD NIH HHS/United States
- R01 AG017241/AG/NIA NIH HHS/United States
- AR40197/AR/NIAMS NIH HHS/United States
- R01 AG015052/AG/NIA NIH HHS/United States
- AG15052/AG/NIA NIH HHS/United States
- R37 AG015052/AG/NIA NIH HHS/United States
- P01 HD023681/HD/NICHD NIH HHS/United States
- R01 AR040197/AR/NIAMS NIH HHS/United States
- AG17241/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources