Identification of NAP1, a regulatory subunit of IkappaB kinase-related kinases that potentiates NF-kappaB signaling - PubMed (original) (raw)
. 2003 Nov;23(21):7780-93.
doi: 10.1128/MCB.23.21.7780-7793.2003.
Yuko Taniguchi, Takashi Kato, Yasuko Narita, Akiko Furuya, Tatsuhiro Ogawa, Hiroaki Sakurai, Takashi Joh, Makoto Itoh, Mireille Delhase, Michael Karin, Makoto Nakanishi
Affiliations
- PMID: 14560022
- PMCID: PMC207563
- DOI: 10.1128/MCB.23.21.7780-7793.2003
Identification of NAP1, a regulatory subunit of IkappaB kinase-related kinases that potentiates NF-kappaB signaling
Fumitaka Fujita et al. Mol Cell Biol. 2003 Nov.
Abstract
The IkappaB kinase (IKK)-related kinase NAK (also known as TBK or T2K) contributes to the activation of NF-kappaB-dependent gene expression. Here we identify NAP1 (for NAK-associated protein 1), a protein that interacts with NAK and its relative IKK epsilon (also known as IKKi). NAP1 activates NAK and facilitates its oligomerization. Interestingly, the NAK-NAP1 complex itself effectively phosphorylated serine 536 of the p65/RelA subunit of NF-kappaB, and this activity was stimulated by tumor necrosis factor alpha (TNF-alpha). Overexpression of NAP1 specifically enhanced cytokine induction of an NF-kappaB-dependent, but not an AP-1-dependent, reporter. Depletion of NAP1 reduced NF-kappaB-dependent reporter gene expression and sensitized cells to TNF-alpha-induced apoptosis. These results define NAP1 as an activator of IKK-related kinases and suggest that the NAK-NAP1 complex may protect cells from TNF-alpha-induced apoptosis by promoting NF-kappaB activation.
Figures
FIG. 1.
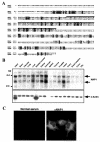
Amino acid sequence and mRNA distribution of NAP1. (A) Sequence similarity between NAP1 and TANK. The predicted amino acid sequences of human NAP1 and TANK are shown in the single-letter code, with dashes representing gaps introduced to optimize alignment. Identical and similar residues are shaded in black and gray, respectively. The minimum region of NAP1 required for binding to NAK is overlined. (B) Tissue distribution of NAP1 mRNA. Polyadenylated RNAs from the indicated human tissues were subjected to Northern blot analysis with NAP1 (upper panel) or β-actin (lower panel) cDNA probes. PBL, peripheral blood leukocytes. The positions of 4.4- and 2.4-kb molecular size standards as well as of NAP1 and β-actin mRNAs are indicated. (C) NAP1 is a cytoplasmic protein. MDAH041 cells cultured on glass coverslips were fixed, permeabilized, and subjected to immunofluorescence analysis with normal mouse serum (left panel) or mouse antibody to NAP1 (αNAP1) (right panel). Immune complexes were detected with Cy3-conjugated goat antibodies to mouse immunoglobulin G. Magnification, ×340.
FIG. 2.
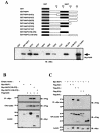
Characterization of the interaction between NAP1 and NAK. (A) In vitro interaction between GST-NAP1 and Myc-NAK. GST fusion proteins containing full-length NAP1 or fragments thereof (10 ng) purified from E. coli were incubated for 1 h on ice with 50 μl of a lysate of Sf9 cells expressing Myc-NAK. The GST pull-down assay was performed as described in Materials and Methods. A portion of Sf9 cell lysatecorresponding to 10% of the input was also immunoblotted with anti-Myc tag antibodies. (B) Association of NAP1 with NAK in transfected mammalian cells. 293T cells were transfected either with 2.0 μg of empty vector or with the indicated combinations of 1.5 μg of wild-type or mutant pcDNA3.1Myc/His6NAP1 and 0.5 μg of pcDNA3FlagNAK. The total amount of transfected DNA was adjusted to 2.0 μg by the addition of empty pcDNA3 vector. Cell extracts were immunoprecipitated (IP) with anti-Myc antibody (αMyc), and the resulting precipitates were disrupted and immunoblotted (IB) with anti-Flag antibodies (upper panel). Whole-cell lysates (10% of the input) were also immunoblotted with anti-Flag antibodies (middle panel). The expression of Myc-NAP1 proteins in the transfected cells was confirmed by immunoblotting the cell lysates with anti-Myc antibodies (lower panel). (C) Association of NAP1 with IKK-related kinases but not with components of the classical IKK complex. 293T cells were transiently transfected with 0.5 μg of pcDNA3FlagNAK, pcDNA3FlagIKKɛ, pcDNA3FlagIKKβ, or pcDNA3FlagIKKγ in the absence or presence of 1.5 μg of pcDNA3.1Myc/His6NAP1, as indicated. The total amount of transfected DNA was adjusted to 2.0 μg by the addition of empty pcDNA3 vector. Cell extracts were immunoprecipitated with antibodies to Myc, and the resulting precipitates were immunoblotted with antibodies to Flag (top panel). Whole-cell lysates (10% of the input) were also directly immunoblotted with antibodies to Flag (middle panel). Expression of Myc-NAP1 was confirmed by immunoblotting with anti-Myc antibodies (bottom panel). Numbers on the left in panels B and C are molecular masses in kilodaltons.
FIG. 3.
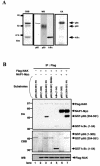
Endogenous NAK-NAP1 complexes. (A) Association of endogenous NAP1 and NAK. Extracts of unstimulated HeLa cells (2 mg of protein) were immunoprecipitated (IP) with either normal rabbit serum (NRS), antibodies to NAP1 (αNAP1), or antibodies to NAK. The resulting immunoprecipitates were disrupted and immunoblotted (WB) with antibodies to NAK (top panel) and to NAP1 (second panel). Whole-cell lysates were also immunoblotted with antibodies to NAK (third panel) or to NAP1 (bottom panel). (B) Association of NAP1 with a high-molecular-weight NAK-containing complex. S100 cytosolic extracts of HeLa cells that had been incubated for 10 min in the absence (nonstimulated) or presence of TNF-α (20 ng/ml) were fractionated on a Superose 6 column. Column fractions (as well as a portion of the input material) were immunoblotted (IB) with antibodies to IKKα, IKKγ, NAK, or NAP1, as indicated. A portion of each fraction was also immunoprecipitated with antibodies to NAK and assayed for associated kinase activity (KA) with GST-p65C as the substrate. The elution of molecular size standards (in kilodaltons) from the column is indicated on the top panels.
FIG. 4.
Activation of NAK by NAP1 in vitro and in vivo. (A) Activation of NAK by NAP1 in vitro. Myc-NAK (10 μl) immunoprecipitated from transfected Sf9 cells with antibodies to Myc was incubated with the indicated amounts of GST-NAP1 and then assayed for kinase activity withGST-IKKβ(132-206) or GST alone as the substrate. The extent of GST-IKKβ(132-206) phosphorylation (shown as the increase relative to that apparent in the absence of GST-NAP1) was determined by phosphorimaging. The autophosphorylation of NAK is also shown. WB, immunoblotting. (B) Activation of NAK by coexpression of NAP1. 293T cells were transiently transfected either with 2.0 μg of empty vector (lanes 1, 4, and 7) or with 0.5 μg of pcDNA3FlagNAK in the absence (lanes 2, 5, and 8) or presence (lanes 3, 6, and 9) of 1.5 μg of pcDNA3.1Myc/His6NAP1. The total amount of DNA transfected was adjusted to 2.0 μg by the addition of empty pcDNA3 vector. Cell extracts were immunoprecipitated with either anti-Flag (αFlag) (lanes 4 to 6) or anti-Myc (lanes 7 to 9), with the amount of extract used for precipitation with anti-Myc being 1.5 times that used for precipitation with anti-Flag. The resulting immunoprecipitates were then assayed for kinase activity (KA) with GST-IKKβ(132-206) as a substrate (bottom panel). Kinase activity is expressed as fold increase relative to that apparent in lane 5. The immunoprecipitates as well as cell lysates were immunoblotted with anti-Flag (middle panel) or anti-Myc (upper panel). IB, immunoblotting. (C) NAP1 facilitates oligomerization of NAK molecules. 293T cells were transiently transfected with 0.2 μg of pcDNA3FlagNAK, with 0.2 μg of pcDNA3.1Myc/His6NAK, or with both in the absence (lanes 1, 3, 4, and 5) or presence (lanes 2, 6, 7, and 8) of 0.2 μg of pcDNA3.1Myc/His6NAP1. The total amount of DNA transfected was adjusted to 2.0 μg by the addition of empty pcDNA3 vector. Cell extracts were immunoprecipitated (IP) with anti-Flag antibody, and the resulting precipitates were immunoblotted with either anti-Myc (top panel) or anti-Flag (second panel) antibodies. Whole-cell lysates (30% input) were immunoblotted with anti-Myc (third panel) or anti-Flag (bottom panel) antibodies to determine the amounts of proteins expressed. Numbers on the left are molecular masses in kilodaltons.
FIG. 5.
NAP1 enhances NF-κB, but not AP-1, activation by either NAK expression, TNF-α, or PMA. (A) 293T cells were cotransfected with 0.5 μg of pcDNA3FlagNAK or empty vector in the absence or presence of 1.25 or 2.5 μg of pcDNA3.1Myc/His6 encoding either full-length (FL)NAP1, NAP1(158-270), or NAP1(Δ158-270), as indicated. The cells were also transfected with 0.1 μg of an NF-κB-dependent luciferase reporter construct and 0.1 μg of a β-galactosidase expression vector (pmiwZ). The total amount of transfected DNA was adjusted to 2.0 μg by addition of empty pcDNA3. The luciferase activity of cell lysates, determined 30 h after transfection, was normalized to the amount of β-galactosidase activity and expressed relative to that of cells transfected with empty vectors. Data are means ± standard deviations (n = 6) of values from a representative experiment. (B) 293T cells that had been cotransfected with the luciferase and β-galactosidase vectors in the absence or presence of pcDNA3.1Myc/His6NAP1 as for panel A were incubated for 6 h in the absence or presence of TNF-α or PMA, as indicated, before determination of luciferase activity. Data are means ± standard deviations (n = 6). (C) 293T cells that had been cotransfected with 0.02 μg of an AP-1-dependent luciferase reporter construct and β-galactosidase vectors in the absence or presence of pcDNA3.1Myc/His6NAP1 as for panel A were incubated for 6 h in the absence or presence of TNF-α or PMA, as indicated, before determination of luciferase activity. Data are means ± standard deviations (n = 6).
FIG. 6.
p65 phosphorylation by NAK and its enhancement by NAP1. (A) NAK phosphorylates the p65 subunit within an NF-κB-IκB ternary complex. Myc-NAK purified from Sf9 cells was assayed for its ability to phosphorylate purified p65-p50-IκBα ternary complexes (KA). The purity and composition of the p65-p50-IκBα ternary complexes were confirmed by both Coomassie brilliant blue (CBB) staining and Western blotting with the indicated antibodies (WB). Numbers on the left are molecular masses in kilodaltons. (B) NAK specifically phosphorylates serine 536 on the carboxyl-terminal region of p65. 293T cells were transfected with pcDNA3FlagNAK alone or together with pcDNA3.1Myc/His6NAP1. Whole-cell extracts were immunoprecipitated (IP) with anti-Flag, and the resultant precipitates were subjected to kinase assay with the indicated GST-p65 or -IκBα proteins as substrates (KA). The amounts of substrates and Flag-NAK were confirmed by CBB staining and Western blotting with anti-Flag, respectively.
FIG. 7.
Reduced TNF-α- or PMA-induced NF-κB activation and increased TNF-α-induced apoptosis after NAP1 depletion. (A) HeLa cells (5 × 105) were transfected three times at 15-h intervals with 5 μg of siRNA targeted to either NAP1 or GFP with the use of Oligofectamine. Forty-five hours after the first transfection, cells were lysed and immunoblotted (IB) with antibodies to NAP1 (αNAP1) (upper panel) or NAK (middle panel). The cell lysates were also immunoprecipitated (IP) with antibodies to NAP1, and the resulting precipitates were immunoblotted with antibodies to NAK (lower panel). (B) HeLa cells (105) were transfected with 1.0 μg of GFP siRNA (open bars) or NAP1 siRNA (solid bars), 0.8μg of NF-κB-dependent luciferase reporter construct, and 0.2 μg of pmiwZ with the use of Lipofectamine 2000. After 48 h, the cells were incubated for 6 h in the absence or presence of TNF-α (10 ng/ml), 20 nM PMA, or IL-1β (20 ng/ml). Luciferase activity in cell lysates was determined and normalized to β-galactosidase activity. Data are expressed as the fold increase over the normalized luciferase activity of nonstimulated, GFP siRNA-transfected cells and are means ± standard deviations (n = 6). (C) HeLa cells were transfected three times with 5 μg of GFP or NAP1 siRNAs as described for panel A. The cells were then incubated for 5 h in the absence or presence of TNF-α (20 ng/ml), after which they were immunoblotted with antibodies specific to either IκBα or its Ser32-phosphorylated form. IKK activity was also determined by immune-complex kinase assay with GST-IκBα as a substrate.(D) HeLa cells transfected as described above were incubated for 5 h in the absence (open bars) or presence (solid bars) of TNF-α (50 ng/ml), after which they were subjected to TUNEL analysis and staining with 4′,6-diamidino-2-phenylindole (DAPI) (upper panel). Magnification, ×36. The percentage of TUNEL-positive cells was determined by counting at least 200 cells in each of three independent fields (lower panel). Data are means ± standard deviations.
References
- Baeuerle, P. A., and D. Baltimore. 1996. NF-κB: ten years after. Cell 87:13-20. - PubMed
- Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274:782-784. - PubMed
- Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-170. - PubMed
- Bonnard, M., C. Mirtsos, S. Suzuki, K. Graham, J. Huang, M. Ng, A. Itie, A. Wakeham, A. Shahinian, W. J. Henzel, A. J. Elia, W. Shillinglaw, T. W. Mak, Z. Cao, and W. C. Yeh. 2000. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-κB-dependent gene transcription. EMBO J. 19:4976-4985. - PMC - PubMed
- Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous