Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads - PubMed (original) (raw)
. 2003 Dec;130(24):5895-902.
doi: 10.1242/dev.00836. Epub 2003 Oct 15.
Affiliations
- PMID: 14561636
- PMCID: PMC4073601
- DOI: 10.1242/dev.00836
Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads
Humphrey H-C Yao et al. Development. 2003 Dec.
Abstract
The developmental fate of primordial germ cells in the mammalian gonad depends on their environment. In the XY gonad, Sry induces a cascade of molecular and cellular events leading to the organization of testis cords. Germ cells are sequestered inside testis cords by 12.5 dpc where they arrest in mitosis. If the testis pathway is not initiated, germ cells spontaneously enter meiosis by 13.5 dpc, and the gonad follows the ovarian fate. We have previously shown that some testis-specific events, such as mesonephric cell migration, can be experimentally induced into XX gonads prior to 12.5 dpc. However, after that time, XX gonads are resistant to the induction of cell migration. In current experiments, we provide evidence that this effect is dependent on XX germ cells rather than on XX somatic cells. We show that, although mesonephric cell migration cannot be induced into normal XX gonads at 14.5 dpc, it can be induced into XX gonads depleted of germ cells. We also show that when 14.5 dpc XX somatic cells are recombined with XY somatic cells, testis cord structures form normally; however, when XX germ cells are recombined with XY somatic cells, cord structures are disrupted. Sandwich culture experiments suggest that the inhibitory effect of XX germ cells is mediated through short-range interactions rather than through a long-range diffusible factor. The developmental stage at which XX germ cells show a disruptive effect on the male pathway is the stage at which meiosis is normally initiated, based on the immunodetection of meiotic markers. We suggest that at the stage when germ cells commit to meiosis, they reinforce ovarian fate by antagonizing the testis pathway.
Figures
Fig. 1
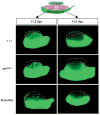
In the absence of germ cells, mesonephric cells can be induced to migrate into 14.5 dpc XX gonads. An 11.5 or 14.5 dpc XX gonad from a wild type (+/+), _KitW/W_-v embryo or busulfan-treated embryo was cultured between an 11.5 dpc XY gonad and an 11.5 dpc GFP mesonephros for 48 hours. Broken blue lines outline the XY components; broken red lines outline the XX components.
Fig. 2
Meiotic germ cells interfere with cord formation. Germ cells and somatic cells were isolated using an immunomagnetic cell sorting system. Somatic cell fractions from 12.5 dpc XY gonads were aggregated with 11.5 dpc XX germ cell fraction (A), 11.5 dpc XY germ cell fraction (B), 14.5 dpc XX somatic fraction (C) or 14.5 dpc XX germ cell fraction (D) and cultured for 48 hours. Formation of testis cords was shown by immunostaining for laminin (green), a component of the basal lamina of testis cords (red arrows).
Fig. 3
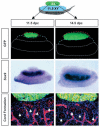
Co-culture experiments suggest that meiotic germ cells inhibit the testis pathway via short-range effects. An 11.5 or 14.5 dpc XX GFP gonad (green) was cultured on top of an 11.5 dpc XY gonad for 48 hours. In situ hybridization for Sox9 (dark purple) or immunocytochemical staining for laminin (blue) and germ cells and vasculature (red), reveal no interference with the testis pathway. Red and blue broken lines outline the XX and XY components, respectively. Broken white lines outline the mesonephros. Arrows indicate testis cord boundaries.
Fig. 4
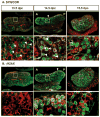
Two meiotic markers, SYN/COR (A) and γH2AX (B), are expressed in an anterior to posterior pattern in 13.5–15.5 dpc XX gonads: SYN/COR and γH2AX (green); germ cells and vasculature (PECAM-1, red). A higher magnification of the region surrounded by the white rectangle is shown below the corresponding image. Consecutive 8 μm sections were stained with each antibody to define the spatial relationship between SYN/COR and γH2AX. Arrows indicate the leading front of staining at 14.5 dpc. Arrowheads indicate the first detection of SYN/COR at 13.5 dpc and the filamentous pattern associated with the assembly of homologous chromatid pairs in zygotene/pachytene at 14.5 dpc. A, anterior; P, posterior.
Fig. 5
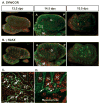
Meiotic germ cells are present transiently in XY gonads in the region of the rete testis. (A) SYN/COR and (B) γH2AX were detected by immunofluorescence (green) in consecutive 8 μm sections; germ cells were labeled with αPECAM1 (red). (C) A higher magnification of the region surrounded by the white rectangle in A. (D) At 15.5 dpc, apoptotic cells in this region were labeled with LysoTracker (magenta); germ cells were labeled with PECAM (red); the basal lamina of testis cords was stained for laminin (green). Arrows indicate germ cells. A, anterior; P, posterior; G, gonad; M, mesonephros; MT, mesonephric tubule; TC, testis cord.
Fig. 6
A model for the antagonistic role of meiotic germ cells on the testis pathway. In normal XX gonads, germ cells follow an intrinsic clock and enter meiosis by 13.5 dpc. In normal XY gonads, expression of Sry at 10.5–12.5 dpc initiates cord formation prior to entry of germ cells into meiosis. Cord formation blocks germ cell entry into meiosis. Molecules on the surface of germ cells or short-range diffusible factors from germ cells may be permissive for testis cord formation only until germ cells commit to meiosis when their surface properties may change. A temporal mismatch experimentally created by combining meiotic germ cells with 12.5 dpc XY somatic cells led to failure of testis cord formation.
Similar articles
- The battle of the sexes: opposing pathways in sex determination.
Yao HH, Tilmann C, Zhao GQ, Capel B. Yao HH, et al. Novartis Found Symp. 2002;244:187-98; discussion 198-206, 253-7. Novartis Found Symp. 2002. PMID: 11990791 Review. - Characterization of mesonephric cells that migrate into the XY gonad during testis differentiation.
Nishino K, Yamanouchi K, Naito K, Tojo H. Nishino K, et al. Exp Cell Res. 2001 Jul 15;267(2):225-32. doi: 10.1006/excr.2001.5238. Exp Cell Res. 2001. PMID: 11426941 - AMH induces mesonephric cell migration in XX gonads.
Ross AJ, Tilman C, Yao H, MacLaughlin D, Capel B. Ross AJ, et al. Mol Cell Endocrinol. 2003 Dec 15;211(1-2):1-7. doi: 10.1016/j.mce.2003.09.021. Mol Cell Endocrinol. 2003. PMID: 14656469 Free PMC article. - FGF9 promotes survival of germ cells in the fetal testis.
DiNapoli L, Batchvarov J, Capel B. DiNapoli L, et al. Development. 2006 Apr;133(8):1519-27. doi: 10.1242/dev.02303. Epub 2006 Mar 15. Development. 2006. PMID: 16540514 - Cell cycle control of germ cell differentiation.
Spiller CM, Koopman P. Spiller CM, et al. Results Probl Cell Differ. 2011;53:269-308. doi: 10.1007/978-3-642-19065-0_13. Results Probl Cell Differ. 2011. PMID: 21630150 Review.
Cited by
- Early depletion of primordial germ cells in zebrafish promotes testis formation.
Tzung KW, Goto R, Saju JM, Sreenivasan R, Saito T, Arai K, Yamaha E, Hossain MS, Calvert MEK, Orbán L. Tzung KW, et al. Stem Cell Reports. 2015 Jan 13;4(1):61-73. doi: 10.1016/j.stemcr.2014.10.011. Epub 2014 Nov 26. Stem Cell Reports. 2015. PMID: 25434820 Free PMC article. - Loss of Mafb and Maf distorts myeloid cell ratios and disrupts fetal mouse testis vascularization and organogenesis†.
Li SY, Gu X, Heinrich A, Hurley EG, Capel B, DeFalco T. Li SY, et al. Biol Reprod. 2021 Oct 11;105(4):958-975. doi: 10.1093/biolre/ioab098. Biol Reprod. 2021. PMID: 34007995 Free PMC article. - Retinoic acid regulates sex-specific timing of meiotic initiation in mice.
Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Koubova J, et al. Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2474-9. doi: 10.1073/pnas.0510813103. Epub 2006 Feb 6. Proc Natl Acad Sci U S A. 2006. PMID: 16461896 Free PMC article. - Sex-specific differences in mouse DMRT1 expression are both cell type- and stage-dependent during gonad development.
Lei N, Hornbaker KI, Rice DA, Karpova T, Agbor VA, Heckert LL. Lei N, et al. Biol Reprod. 2007 Sep;77(3):466-75. doi: 10.1095/biolreprod.106.058784. Epub 2007 Jun 13. Biol Reprod. 2007. PMID: 17567962 Free PMC article. - Retinoic Acid and Germ Cell Development in the Ovary and Testis.
Endo T, Mikedis MM, Nicholls PK, Page DC, de Rooij DG. Endo T, et al. Biomolecules. 2019 Nov 24;9(12):775. doi: 10.3390/biom9120775. Biomolecules. 2019. PMID: 31771306 Free PMC article. Review.
References
- Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129:1155–1164. - PubMed
- Albrecht KH, Capel B, Washburn LL, Eicher EM. Defective mesonephric cell migration is associated with abnormal testis cord development in C57BL/6J XYMus domesticus mice. Dev Biol. 2000;225:26–36. - PubMed
- Bradbury MW. Testes of XX in equilibrium with XY chimeric mice develop from fetal ovotestes. Dev Genet. 1987;8:207–218. - PubMed
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. - PubMed
- Burgoyne P, Palmer S. The genetics of XY sex reversal in the mouse and other mammals. Semin Dev Biol. 1991;2:277–284.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases