Long-chain acyl-CoA esters and phosphatidylinositol phosphates modulate ATP inhibition of KATP channels by the same mechanism - PubMed (original) (raw)
Long-chain acyl-CoA esters and phosphatidylinositol phosphates modulate ATP inhibition of KATP channels by the same mechanism
Dirk Schulze et al. J Physiol. 2003.
Abstract
Phosphatidylinositol phosphates (PIPs, e.g. PIP2) and long-chain acyl-CoA esters (e.g. oleoyl-CoA) are potent activators of KATP channels that are thought to link KATP channel activity to the cellular metabolism of PIPs and fatty acids. Here we show that the two types of lipid act by the same mechanism: oleoyl-CoA potently reduced the ATP sensitivity of cardiac (Kir6.2/SUR2A) and pancreatic (Kir6.2/SUR1) KATP channels in a way very similar to PIP2. Mutations (R54Q, R176A) in the C- and N-terminus of Kir6.2 that greatly reduced the PIP2 modulation of ATP sensitivity likewise reduced the modulation by oleoyl-CoA, indicating that the two lipids interact with the same site. Polyvalent cations reduced the effect of oleoyl-CoA and PIP2 on the ATP sensitivity with similar potency suggesting that electrostatic interactions are of similar importance. However, experiments with differently charged inhibitory adenosine phosphates (ATP4-, ADP3- and 2'(3')-O-(2,4,6-trinitrophenyl)adenosine 5'-monophosphate (TNP-AMP2-)) and diadenosine tetraphosphate (Ap4A5-) ruled out a mechanism where oleoyl-CoA or PIP2 attenuate ATP inhibition by reducing ATP binding through electrostatic repulsion. Surprisingly, CoA (the head group of oleoyl-CoA) did not activate but inhibited KATP channels (IC50 = 265 +/- 33 muM). We provide evidence that CoA and diadenosine polyphosphates (e.g. Ap4A) are ligands of the inhibitory ATP-binding site on Kir6.2.
Figures
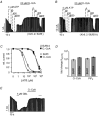
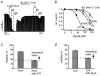
Figure 1. Oleoyl-CoA and PIP2 reduce ATP inhibition of cardiac and pancreatic KATP channels with similar potency
Pancreatic (Kir6.2/SUR1) and cardiac (Kir6.2/SUR2A) KATP currents were measured in giant inside-out patches excised from Xenopus oocytes at -90 mV; inward currents are shown as upward deflections. A, effects of ATP and oleoyl-CoA (O.-CoA) on pancreatic KATP channels at the concentrations indicated. B, effects of ATP and oleoyl-CoA on cardiac KATP channels. C, from experiments such as those in A and B, concentration-response curves were created for ATP inhibition before (□, ○) and after (▪, •) application of 20 μM oleoyl-CoA for 30 s. Continuous lines represent fits to a standard Hill equation: I/_I_max= 1/(1 + ([ATP]/_K_i(ATP)_n_H)), error bars represent ±S.E.M. rel. current, relative current. D, the bars represent the fold change ±S.E.M. of the _K_i(ATP) for cardiac and pancreatic KATP channels upon application of oleoyl-CoA (as in C) or PIP2 (20 μM for 30 s). E, effects of glibenclamide (Glib) and 10 μM oleoyl-CoA on cardiac KATP channels.
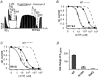
Figure 2. R54Q and R176A reduce oleoyl-CoA modulation of ATP inhibition
A, ATP sensitivity of R176A (Kir6.2/SUR2A) currents measured subsequent to run down (see magnification) and after application of 20 μM oleoyl-CoA for 30 s. B, concentration-response curves from experiments such as that in A; circles represent data before (○) and after (•) application of oleoyl-CoA; WT concentration-response curves (dashed lines) are shown for comparison. C, concentration-response curves for R54Q channels before (○) and after (•) application of oleoyl-CoA. WT concentration-response curves (dashed lines) are shown for comparison. D, the bars represent the fold change ±S.E.M. for _K_i(ATP) upon oleoyl-CoA application for WT, R176A and R54Q channels.
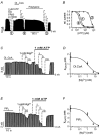
Figure 3. Oleoyl-CoA reduce the ATP sensitivity by an electrostatic mechanism
A, effects of 20 μM oleoyl-CoA, 2 μg ml−1 polylysine and ATP on cardiac KATP channels. B, from the experiment in A, concentration-response curves for ATP inhibition were obtained before oleoyl-CoA (1), after oleoyl-CoA (2) and after polylysine (3). C, effects of Mg2+ in the presence and absence of 1 mM ATP on KATP channels subsequent to the application of 20 μM oleoyl-CoA. D, the amount of ATP inhibition was used to estimate the K_i(ATP) using the Hill equation for ATP inhibition (Fig. 1_C) with a Hill coefficient of 1.8. A plot of these _K_i(ATP) values against the respective Mg2+ concentrations resulted in a concentration-response curve that was fitted to a standard Hill function with an IC50(Mg2+) of 1.3 ± 0.2 mM and Hill coefficient of 1. E, effects of Mg2+ in the presence and absence of 1 mM ATP on KATP channels subsequent to the application of 20 μM PIP2. The amount of ATP inhibition was used to estimate the K_i(ATP) using the Hill equation for ATP inhibition (Fig. 1_C) with a Hill coefficient of 1.8. A plot of these _K_i(ATP) values against the respective Mg2+ concentrations resulted in a concentration-response curve that was fitted to a standard Hill function with an IC50(Mg2+) of 1.5 ± 0.2 mM and Hill coefficient of 1.
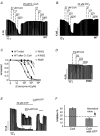
Figure 4. Effects of oleoyl-CoA and PIP2 on inhibition of KATP current by various (di)adenosine phosphates
A, effect of application of 750 μM TNP-AMP (AMP), 2000 μM ADP, 15 μM ATP, 15 μM Ap4A and 4 μM oleoyl-CoA on cardiac KATP channels as indicated. B, same concentrations as in A, but with application of 20 μM PIP2 instead of oleoyl-CoA. C, inhibition of KATP channels by (di)adenosine phosphates at the concentrations indicated.
Figure 5. CoA causes inhibition by interacting with the ATP-binding site on Kir6.2
A, CoA inhibited cardiac KATP currents in a concentration-dependent manner and application of 20 μM oleoyl-CoA or 20 μM PIP2 (B) largely reduced CoA inhibition. C, concentration-response curves for CoA inhibition for WT, R50E, R54Q and K185Q channels with fits to standard Hill equations with K_i(ATP) values of 265 ± 33 μM (WT), 6 ± 0.5 mM (R50E), 92 ± 6 μM (R54Q) and 1.9 ± 0.2 mM (K185Q). D, inhibition of R50E channels by different concentrations of CoA. E, inhibition of KATP currents by CoA in the presence and absence of ATP at the concentrations indicated; magnification shows CoA inhibition in the presence of 50 μM ATP. F, from experiments such as that in E, current inhibition was determined for 333 μM oleoyl-CoA in the absence and presence of 50 μM ATP and plotted as means ± S.E.M. The dashed line represents the expected theoretical value for current inhibition by CoA in the presence of 50 μM ATP, assuming direct competition between CoA and ATP. To obtain this value, the amount of CoA inhibition (in the absence of ATP) was used to calculate the ATP concentration (14.2 μM) necessary to produce the same inhibition using a concentration-response curve as in Fig. 1_C. Accordingly, the theoretical value represents the expected increase in ATP inhibition produced by a rise in the ATP concentration from 50 μM to (50 + 14.2) μM.
Figure 6. Ap4A and ATP compete for the same site to inhibit KATP channels
A, Ap4A inhibited cardiac KATP currents in a concentration-dependent manner and application of 20 μM oleoyl-CoA greatly reduced Ap4A inhibition. B, concentration-response curves for Ap4A inhibition of WT, R50E and K185Q channels, and WT channels after oleoyl-CoA (A); fits to standard Hill equations with K_i(Ap4A) values of 10 ± 1 μM (WT), 438 ± 77 μM (R50E) and 200 ± 35 μM (K185Q). C, inhibition of KATP currents by 16 μM Ap4A in the presence and absence of 40 μM ATP is plotted as means ± S.E.M. The dashed line represents the expected theoretical value for current inhibition by 16 μM Ap4A in the presence of 40 μM ATP assuming direct competition between Ap4A and ATP. To obtain this value, the amount of Ap4A inhibition (in the absence of ATP) was used to calculate the ATP concentration (14.5 μM) necessary to produce the same inhibition using a concentration-response curve as in Fig. 1_C. Accordingly, the theoretical value represents the expected increase in ATP inhibition produced by a rise in the ATP concentration from 40 μM to (40 + 14.5) μM. D, inhibition of KATP currents by 333 μM CoA in the presence and absence of 40 μM Ap4A is plotted as means ± S.E.M. The dashed line represents the expected theoretical value for current inhibition by 333 μM CoA in the presence of 40 μM Ap4A assuming direct competition between CoA and Ap4A (the theoretical value was calculated as described in C).
Similar articles
- Long chain coenzyme A esters activate the pore-forming subunit (Kir6. 2) of the ATP-regulated potassium channel.
Bränström R, Leibiger IB, Leibiger B, Corkey BE, Berggren PO, Larsson O. Bränström R, et al. J Biol Chem. 1998 Nov 20;273(47):31395-400. doi: 10.1074/jbc.273.47.31395. J Biol Chem. 1998. PMID: 9813050 - Single residue (K332A) substitution in Kir6.2 abolishes the stimulatory effect of long-chain acyl-CoA esters: indications for a long-chain acyl-CoA ester binding motif.
Bränström R, Leibiger IB, Leibiger B, Klement G, Nilsson J, Arhem P, Aspinwall CA, Corkey BE, Larsson O, Berggren PO. Bränström R, et al. Diabetologia. 2007 Aug;50(8):1670-7. doi: 10.1007/s00125-007-0697-x. Epub 2007 May 24. Diabetologia. 2007. PMID: 17522836 - Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA.
Gribble FM, Proks P, Corkey BE, Ashcroft FM. Gribble FM, et al. J Biol Chem. 1998 Oct 9;273(41):26383-7. doi: 10.1074/jbc.273.41.26383. J Biol Chem. 1998. PMID: 9756869 - ATP sensitive potassium channel and myocardial preconditioning.
Day YJ, Gao Z, Tan PC, Linden J. Day YJ, et al. Acta Anaesthesiol Sin. 1999 Sep;37(3):121-31. Acta Anaesthesiol Sin. 1999. PMID: 10609345 Review. - A view of sur/KIR6.X, KATP channels.
Babenko AP, Aguilar-Bryan L, Bryan J. Babenko AP, et al. Annu Rev Physiol. 1998;60:667-87. doi: 10.1146/annurev.physiol.60.1.667. Annu Rev Physiol. 1998. PMID: 9558481 Review.
Cited by
- Cardiac and vascular KATP channels in rats are activated by endogenous epoxyeicosatrienoic acids through different mechanisms.
Lu T, Ye D, Wang X, Seubert JM, Graves JP, Bradbury JA, Zeldin DC, Lee HC. Lu T, et al. J Physiol. 2006 Sep 1;575(Pt 2):627-44. doi: 10.1113/jphysiol.2006.113985. Epub 2006 Jun 22. J Physiol. 2006. PMID: 16793897 Free PMC article. - A novel KCNJ11 mutation associated with congenital hyperinsulinism reduces the intrinsic open probability of beta-cell ATP-sensitive potassium channels.
Lin YW, MacMullen C, Ganguly A, Stanley CA, Shyng SL. Lin YW, et al. J Biol Chem. 2006 Feb 3;281(5):3006-12. doi: 10.1074/jbc.M511875200. Epub 2005 Dec 6. J Biol Chem. 2006. PMID: 16332676 Free PMC article. - Inhibition of cholinergic potentiation of insulin secretion from pancreatic islets by chronic elevation of glucose and fatty acids: Protection by casein kinase 2 inhibitor.
Doliba NM, Liu Q, Li C, Chen P, Liu C, Naji A, Matschinsky FM. Doliba NM, et al. Mol Metab. 2017 Oct;6(10):1240-1253. doi: 10.1016/j.molmet.2017.07.017. Epub 2017 Aug 4. Mol Metab. 2017. PMID: 29031723 Free PMC article. - Early Effects of Metabolic Syndrome on ATP-Sensitive Potassium Channels from Rat Pancreatic Beta Cells.
Cruz-Cruz I, Bernate-Obando G, Larqué C, Escalona R, Pinto-Almazán R, Velasco M. Cruz-Cruz I, et al. Metabolites. 2022 Apr 18;12(4):365. doi: 10.3390/metabo12040365. Metabolites. 2022. PMID: 35448552 Free PMC article. - Modulation of Potassium Channel Activity in the Balance of ROS and ATP Production by Durum Wheat Mitochondria-An Amazing Defense Tool Against Hyperosmotic Stress.
Trono D, Laus MN, Soccio M, Alfarano M, Pastore D. Trono D, et al. Front Plant Sci. 2015 Dec 1;6:1072. doi: 10.3389/fpls.2015.01072. eCollection 2015. Front Plant Sci. 2015. PMID: 26648958 Free PMC article. Review.
References
- Baukrowitz T, Fakler B. K(ATP) channels: Linker between phospholipid metabolism and excitability. Biochem Pharmacol. 2000;60:735–740. - PubMed
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. - PubMed
- Branstrom R, Leibiger IB, Leibiger B, Corkey BE, Berggren PO, Larsson O. Long chain coenzyme A esters activate the pore-forming subunit (Kir6. 2) of the ATP-regulated potassium channel. J Biol Chem. 1998;273:31395–31400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources