Autoimmune cardiomyopathy and heart block develop spontaneously in HLA-DQ8 transgenic IAbeta knockout NOD mice - PubMed (original) (raw)
Autoimmune cardiomyopathy and heart block develop spontaneously in HLA-DQ8 transgenic IAbeta knockout NOD mice
John F Elliott et al. Proc Natl Acad Sci U S A. 2003.
Abstract
A line of nonobese diabetic (NOD) mice expressing the human diabetes-associated HLA-DQ8 transgene in the absence of mouse IAbeta failed to show spontaneous insulitis or diabetes, but rather developed dilated cardiomyopathy, leading to early death from heart failure. Pathology in these animals results from an organ- and cell-specific autoimmune response against normal cardiomyoctes in the atrial and ventricular walls, as well as against very similar myocytes present in the outermost muscle layer surrounding the pulmonary veins. Progression of the autoimmune process could be followed by serial ECG measurements; irradiation of young animals significantly delayed disease progression, and this effect could be reversed by adoptive transfer of splenocytes taken from older animals with complete heart block. Disease progression could also be blocked by cyclosporin A treatment, but was accelerated by injection of complete Fruend's adjuvant. The constellation of findings of spontaneously arising destructive focal lymphocytic infiltrates within the myocardium, rising titers of circulating anticardiac autoantibodies, dilation of the cardiac chambers, and gradual progression to end-stage heart failure bears a striking resemblance to what is seen in humans with idiopathic dilated cardiomyopathy, a serious and often life-threatening medical condition. This transgenic strain provides a highly relevant animal model for human autoimmune myocarditis and postinflammatory dilated cardiomyopathy.
Figures
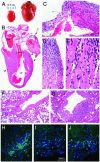
Fig. 1.
(A) Photograph of intact heart from a 22-week-old preterminal DQ8+/+,_IA_β-/- female NOD mouse (Right; 0.57 g) compared with that of a healthy age- and sex-matched NOD/LtJ animal (Left; 0.14 g). (_B_-E) Histology of a typical end-stage diseased heart, stained with hematoxylin and eosin (representative of n = 12 animals). (B) Areas indicated by arrows are shown at higher power in C and D. RA and LV indicate right atrium and left ventricle, respectively. A large organized thrombus (T) fills most of the left atrium. Atrial walls are extremely thin, and the muscle has been replaced with fibrous tissue in most places. Residual patches of dying atrial cardiomyocytes stain bright pink (C) and are associated with mononuclear cell infiltrates. Dense focal mononuclear cell infiltrates associated with myocyte damage can be seen in the ventricular endocardium (D; and at higher magnification in E) and in a perivascular location on the epicardial surface (B, arrowheads). The perivascular mononuclear cells were characterized further by indirect immunofluorescence. The majority of cells showed surface or cytoplasmic IgG expression consistent with B lymphocytes and plasma cells (H; light blue-green staining cells); smaller numbers of CD4+ and CD8+ T cells were also clearly present (I and J, respectively). V, epicardial blood vessel. Note that in _H_-J all cellular nuclei fluoresce dark blue because of 4′,6-diamidino-2-phenylindole counter-staining, and muscle proteins within the cardiomyocytes fluoresce dark green. F and G show, respectively, pulmonary veins from a normal 5-week-old NOD/LtJ and a 5-week-old DQ8+/+,_IA_β-/- animal. (G) A dense collection of mononuclear cells is seen to be associated with the destruction of the ensheathing cardiomyocyte layer on the lower right aspect of the pulmonary vein. Note that during fixation the surrounding lung was less well inflated in G than in F. (Magnifications: B, ×3; C and D and _F_-J, ×100; E, ×400).
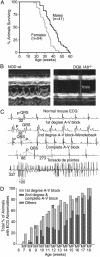
Fig. 2.
(A) Survival curves for male and female DQ8+/+,_IA_β-/- mice. Animals were monitored from 12 weeks of age onward; severely ill animals were killed. (B) M-mode echocardiogram from a healthy 20-week-old female NOD/Lt mouse (Left) versus a preterminal DQ8+/+,_IA_β-/- NOD (Right). The location of the anterior and posterior endocarial walls is defined by the brackets, with time along the y axis. The NOD heart has a normal anteroposterior diameter and is seen to contract normally over time, whereas the diseased heart is significantly dilated and barely contracts (representative of n = 8 animals). Echocardiography also revealed other abnormalities such as mitrial regurgitation in older animals (data not shown). (C) Representative ECG traces taken from a normal mouse (NOD/LtJ; top trace) and DQ8+/+,_IA_β-/- NOD animals of various ages (lower four traces). P waves and QRS complexes are indicated; numbers indicate lengths of various intervals in msec. Note that the time scale in the bottom trace differs from the rest. (D) Summary of ECG findings for male (M) and female (F) DQ8+/+,_IA_β-/- NOD mice of various ages.
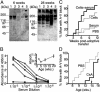
Fig. 3.
(A) Identical Western blots made with heart muscle proteins and probed with sera from two different DQ8+/+,_IA_β-/- NOD animals (Left, 6-week-old animal without heart block, 1:10,000 dilution; Right, 28-week-old animal with complete AV block, 1:50,000 dilution). Lanes contain muscle proteins extracted from: 1, mouse atria; 2, mouse ventricles; 3, mouse skeletal muscle or purchased commercially from Sigma; 4, purified porcine skeletal myosin; and 5, purified porcine cardiac myosin. (B) Titration curves for anticardiac myosin antibodies present in the sera of DQ8+/+,_IA_β-/- NOD mice of various ages. Sera were pooled from animals ages 2.5 weeks (n = 9; ▴), 5 weeks (n = 7; ⋄), 10 weeks (n = 7; •), and 20 weeks (n = 6; ○). As a control, values for pooled sera from DQ8+/+,_IA_βg7+/+ NOD mice (n = 5; □) are also shown. A number of other control strains (NOD/Lt, IAβ-/-NOD, C57BL/6) were similarly negative. Total serum IgG concentrations in older preterminal DQ8+/+,_IA_β-/- NOD animals were somewhat higher (up to 4-fold increased) compared with those found in control strains. ELISA plates were coated with porcine cardiac myosin, incubated with various dilutions of pooled antisera and then secondary antibodies, and developed with horseradish peroxidase. The data are expressed as mean of pooled sera tested in triplicate. (Inset) Data replotted as absorbance versus age for pooled sera at the 1:100,000 dilution. (C) Incidence of heart block in irradiated animals that received adoptive transfer of splenocytes, cells (n = 12), serum (n = 12), both agents (n = 17), or PBS only (n = 20). All animals were irradiated at 5 weeks of age, injected as indicated, and then followed with weekly ECGs. The percentage of animals showing first-degree or complete heart block at various weeks posttransfer are indicated. The serum and PBS groups were not statistically different, nor were the cells vs. cells plus serum groups. At 11 and 12 weeks, the cells group statistically differed from the PBS group with P = 0.03. Similarly, at 11 and 12 weeks, the cells plus serum group differed from the PBS group with P = 0.001. Fisher's exact test was used to calculate two-tailed P values. (D) Heart block in animals receiving CsA injections vs. PBS. Beginning at weaning, animals were given daily injections of CsA (10 mg/kg s.c) or PBS (n = 10 animals in each group). Values indicate the percentage of animals with first-degree or complete heart block. CsA injections were discontinued at 15 weeks of age (indicated by arrow). At 14 and 15 weeks of age, the two groups were statistically different (P = 0.023) by Fisher's exact test.
Similar articles
- CD4 T cells play major effector role and CD8 T cells initiating role in spontaneous autoimmune myocarditis of HLA-DQ8 transgenic IAb knockout nonobese diabetic mice.
Hayward SL, Bautista-Lopez N, Suzuki K, Atrazhev A, Dickie P, Elliott JF. Hayward SL, et al. J Immunol. 2006 Jun 15;176(12):7715-25. doi: 10.4049/jimmunol.176.12.7715. J Immunol. 2006. PMID: 16751419 - Spontaneous autoimmune myocarditis and cardiomyopathy in HLA-DQ8.NODAbo transgenic mice.
Taneja V, David CS. Taneja V, et al. J Autoimmun. 2009 Nov-Dec;33(3-4):260-9. doi: 10.1016/j.jaut.2009.09.005. Epub 2009 Oct 7. J Autoimmun. 2009. PMID: 19811893 Free PMC article. - A spontaneous model for autoimmune myocarditis using the human MHC molecule HLA-DQ8.
Taylor JA, Havari E, McInerney MF, Bronson R, Wucherpfennig KW, Lipes MA. Taylor JA, et al. J Immunol. 2004 Feb 15;172(4):2651-8. doi: 10.4049/jimmunol.172.4.2651. J Immunol. 2004. PMID: 14764740 - Genetically determined myocarditis: clinical presentation and immunological characteristics.
Caforio AL, Iliceto S. Caforio AL, et al. Curr Opin Cardiol. 2008 May;23(3):219-26. doi: 10.1097/HCO.0b013e3282fbf572. Curr Opin Cardiol. 2008. PMID: 18382209 Review. - The protective effect of IFN-gamma in experimental autoimmune diseases: a central role of mycobacterial adjuvant-induced myelopoiesis.
Matthys P, Vermeire K, Heremans H, Billiau A. Matthys P, et al. J Leukoc Biol. 2000 Oct;68(4):447-54. J Leukoc Biol. 2000. PMID: 11037964 Review.
Cited by
- Dilated cardiomyopathy.
Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A, Mazzanti A, McMurray J, Priori SG. Schultheiss HP, et al. Nat Rev Dis Primers. 2019 May 9;5(1):32. doi: 10.1038/s41572-019-0084-1. Nat Rev Dis Primers. 2019. PMID: 31073128 Free PMC article. Review. - Impaired thymic tolerance to α-myosin directs autoimmunity to the heart in mice and humans.
Lv H, Havari E, Pinto S, Gottumukkala RV, Cornivelli L, Raddassi K, Matsui T, Rosenzweig A, Bronson RT, Smith R, Fletcher AL, Turley SJ, Wucherpfennig K, Kyewski B, Lipes MA. Lv H, et al. J Clin Invest. 2011 Apr;121(4):1561-73. doi: 10.1172/JCI44583. Epub 2011 Mar 23. J Clin Invest. 2011. PMID: 21436590 Free PMC article. - FTY720 (Gilenya) treatment prevents spontaneous autoimmune myocarditis and dilated cardiomyopathy in transgenic HLA-DQ8-BALB/c mice.
Boldizsar F, Tarjanyi O, Olasz K, Hegyi A, Mikecz K, Glant TT, Rauch TA. Boldizsar F, et al. Cardiovasc Pathol. 2016 Sep-Oct;25(5):353-61. doi: 10.1016/j.carpath.2016.05.003. Epub 2016 May 17. Cardiovasc Pathol. 2016. PMID: 27288745 Free PMC article. - What can the HLA transgenic mouse tell us about autoimmune diabetes?
Wong FS, Wen L. Wong FS, et al. Diabetologia. 2004 Sep;47(9):1476-87. doi: 10.1007/s00125-004-1505-5. Epub 2004 Sep 2. Diabetologia. 2004. PMID: 15349728 Review. - The effects of aerobic interval training on the left ventricular morphology and function of VLCAD-deficient mice.
Riggs CE Jr, Michaelides MA, Parpa KM, Smith-Blair NJ. Riggs CE Jr, et al. Eur J Appl Physiol. 2010 Nov;110(5):915-23. doi: 10.1007/s00421-010-1578-4. Epub 2010 Jul 17. Eur J Appl Physiol. 2010. PMID: 20640438
References
- Dec, G. W. & DeSanctis, R. W. (1998) in Scientific American Medicine, eds. Dale, D. C. & Federman, D. D. (Scientific American, New York), pp. XIV-1.
- Manolio, T. A., Baughman, K. L., Rodeheffer, R., Pearson, T. A., Bristow, J. D., Michels, V. V., Abelmann, W. H. & Harlan, W. R. (1992) Am. J. Cardiol. 69, 1458-1466. - PubMed
- Hosenpud, J. D. & Jarcho, J. A. (2000) in Congestive Heart Failure, eds. Hosenput, J. D. & Greenberg, B. H. (Lippincott, Philadelphia), pp. 281-284.
- Cetta, F. & Michels, V. V. (1995) Ann. Med. 27, 169-173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials