Ionic mechanisms of burst firing in dissociated Purkinje neurons - PubMed (original) (raw)
Ionic mechanisms of burst firing in dissociated Purkinje neurons
Andrew M Swensen et al. J Neurosci. 2003.
Abstract
Cerebellar Purkinje neurons have intrinsic membrane properties that favor burst firing, seen not only during complex spikes elicited by climbing fiber input but also with direct electrical stimulation of cell bodies. We examined the ionic conductances that underlie all-or-none burst firing elicited in acutely dissociated mouse Purkinje neurons by short depolarizing current injections. Blocking voltage-dependent calcium entry by cadmium or replacement of external calcium by magnesium enhanced burst firing, but it was blocked by cobalt replacement of calcium, probably reflecting block of sodium channels. In voltage-clamp experiments, we used the burst waveform of each cell as a voltage command and used ionic substitutions and pharmacological manipulations to isolate tetrodotoxin (TTX)-sensitive sodium current, P-type and T-type calcium current, hyperpolarization-activated cation current (Ih), voltage-activated potassium current, large-conductance calcium-activated potassium current, and small-conductance calcium-activated potassium (SK) current. Measured near the middle of the first interspike interval, TTX-sensitive sodium current carried the largest inward current, and T-type calcium current was also substantial. Current through P-type channels was large immediately after a spike but decayed rapidly. These inward currents were opposed by substantial components of voltage-dependent and calcium-dependent potassium current. Termination of the burst is caused partly by decay of sodium current, together with a progressive buildup of SK current after the first interspike interval. Although burst firing depends on the net balance between multiple large currents flowing after a spike, it is surprisingly robust, probably reflecting complex interactions between the exact voltage waveform and voltage and calcium dependence of the various currents.
Figures
Figure 9.
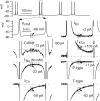
Spontaneous bursts recorded extracellularly in cell-attached mode. A, Spontaneous two and three spike bursts recorded extracellularly in cell-attached mode from a dissociated Purkinje neuron. B, A spontaneous burst recorded in cell-attached mode extracellularly (top panel) and an elicited burst recorded from the same cell in whole-cell current-clamp mode after breaking through the membrane. Spontaneous and triggered bursts showed very similar time courses and number of spikes per burst. In cell-attached recordings, pipettes contained standard intracellular solution with high potassium; large upward currents reflect ionic current flowing from the pipette into the cell when potassium channels in the patch are open.
Figure 3.
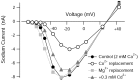
Effects of inorganic calcium channel blockers on the TTX-sensitive sodium current in Purkinje neurons. Plot of peak sodium current versus test potential for a representative cell in which the effects of various calcium channels blockers were assayed. Sodium currents were measured in reduced NaCl (50 m
m
) using TTX subtraction. Holding voltage was -85 mV. For cobalt and magnesium, 2 m
m
CaCl2 was completely replaced by equimolar CoCl2 or MgCl2. Cadmium was applied as 0.3 m
m
CdCl2, with CaCl2 reduced to 1.7 m
m
to keep the total divalent concentration constant.
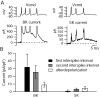
Figure 12.
Large-conductance and small-conductance calcium-activated potassium currents flowing during elicited bursts. A, BK current (left) was obtained as the current sensitive to 150 n
m
iberiotoxin. SK current (right) was obtained as the current sensitive to 30 n
m
scyllatoxin. B, Collected results for the size of BK and SK currents during bursts of three or more spikes (n = 7). BK current was obtained as current sensitive to 100-150 n
m
iberiotoxin, and SK current was obtained as current sensitive to 30 n
m
scyllatoxin (4 cells) or 200 n
m
apamin (3 cells). For each burst, values for the first and second interspike intervals were averaged over a 1 msec window in the middle of the interspike interval (and normalized to the capacitance of the cell). Values for the afterdepolarization (ADP) were averaged over a 1 msec time window around the peak of the ADP. Error bars represent mean ± SD.
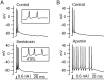
Figure 13.
Effects of BK and SK block on elicited bursts. A, The BK blocker iberiotoxin (100 n
m
) shortened the initial interspike interval and increased the burst from two spikes to three. Inset, Expanded time scale. B, The SK blocker apamin (200 n
m
) increased the burst from two spikes to nine spikes.
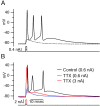
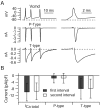
Figure 1.
Burst firing in dissociated Purkinje neurons. A, All-or-none burst elicited in response to a brief (1 msec) depolarizing current injection. Dashed trace shows passive response to current injection to just below the voltage threshold. B, Effect of 2 μ
m
TTX. TTX completely blocked the active response elicited by a moderate suprathreshold current injection (blue), but with a very large (3 nA) current injection for 1 msec (red), the response during the stimulus (passive response to +30 mV, followed by repolarization caused by activation of potassium conductance) was followed by an afterdepolarization.
Figure 2.
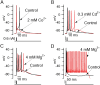
Differential effects of calcium channel blockers on bursting behavior. A, Replacing CaCl2 with equimolar (2 m
m
) CoCl2 inhibited bursting. B, Blocking calcium current with 0.3 m
m
CdCl2 (CaCl2 was reduced to 1.7 m
m
to keep the total divalent concentration constant) enhanced bursting. C, Replacing 2 m
m
CaCl2 by 2 m
m
MgCl2 enhanced bursting. D, In some cases, long-lasting plateaus with fast spikes superimposed were triggered when calcium currents were blocked by cadmium or magnesium.
Figure 4.
Strategy and solutions for isolating various ionic currents using the action potential clamp. Bursts were first recorded in current clamp. The amplifier was then switched into voltage clamp, and the burst waveform was used as the voltage command. Sodium current was isolated as TTX-sensitive current, with 1 μ
m
TTX applied in a solution with reduced (50 m
m
) sodium to improve voltage control and 105 m
m
TEA to block potassium currents. Calcium currents were obtained by subtracting the currents elicited in zero calcium (magnesium replacement) from the currents elicited in full calcium (2 m
m
), with a background of 105 m
m
TEA to block calcium-activated potassium currents. Potassium currents were derived from TEA subtractions with calcium (KCa + Kv) or without calcium (Kv). Calcium-dependent potassium current was then obtained by subtracting the voltage-dependent potassium current from the total potassium current. Changes in solution are indicated by bold, underlined components.
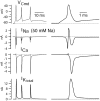
Figure 5.
Sodium, calcium, and (total) potassium current elicited by a burst waveform. Right column shows first spike on an expanded time scale. Solution changes are as in Figure 4. Sodium currents were recorded using an external solution containing 50 m
m
NaCl (and 105 m
m
TEA). The reversal potential for sodium channel current with this solution is approximately +28 mV, so that sodium channel current is outward at the peaks of the action potentials (+44 mV for the first spike), which were recorded in current clamp using 155 m
m
sodium.
Figure 6.
Ionic currents flowing during the interspike intervals of evoked burst. A, Solution changes as in Figure 4. All panels are from the same cell. Values are averaged over a 1 msec window (gray boxes) starting 1.5 msec after the post-spike trough. Sodium currents were recorded in 50 m
m
NaCl. Total calcium-activated potassium current contained an apparent transient inward current during the spike (truncated in the figure). This is likely caused by an effect of Ca replacement on the timing of the voltage-dependent potassium current during the spike (less effective screening of surface charge by Mg results in a hyperpolarizing shift of voltage dependence of _I_Kv and more rapid activation during the spike). B, Cesium-sensitive current (_I_h) measured in another cell. Top panel shows voltage waveform during the burst in this cell; bottom panel shows current sensitive to 1 m
m
Cs+, applied with a background of normal external sodium, with 1 μ
m
TTX to block voltage-dependent sodium currents and 4 m
m
TEA to block potassium currents. As expected for _I_h, which has a reversal potential near -30 mV in physiological solutions, the Cs-sensitive current was inward during the interspike intervals but outward during the spikes.
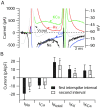
Figure 7.
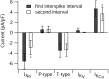
A, Ionic currents during the first interspike interval on an expanded current scale to show the differing time course of the various currents during the interspike interval. Same cell as in Figures 5 and 6. Gray represents voltage during burst waveform (right-hand _y_-axis). Black represents sodium current. Blue represents calcium current. Red represents purely voltage-activated potassium current. Green represents calcium-activated potassium current. B, Collected results for magnitude of the various ionic currents during the first and second interspike intervals measured as in Figure 6, averaged over a 1 msec window starting 1.5 msec after the trough and normalized to the capacitance of the cell. Bars show mean ± SD. *p < 0.001; paired t test. The data for sodium and calcium currents are from 10 Purkinje neurons, and the data from potassium currents are from 8 of the same neurons in which a full set of solution exchanges was possible.
Figure 8.
P-type and T-type calcium currents during elicited bursts. A, Time course of P-type and T-type calcium channel currents during a burst. P-type current was defined as current (carried by 2 m
m
calcium) blocked by 200 n
m
ω-Aga-IVA, applied in an external solution of 50 m
m
NaCl, 105 TEACl, 4 m
m
KCl, 2 m
m
CaCl2, 2 m
m
MgCl2, 10 m
m
HEPES, pH 7.4 with NaOH, with 1 μ
m
TTX. T-type current was defined as the current blocked by 10 μ
m
mibefradil added in the continuous presence of ω-Aga-IVA. B, Collected results for magnitude of total calcium current, P-type calcium current, and T-type calcium current. Currents were averaged over a 1 msec window starting 1.5 msec after the trough and normalized to the capacitance of the cell. Bars show mean ± SD. Total calcium current was determined by Mg2+ replacement of calcium; then, in the same cells, P-type current and T-type current were determined as in A. The sum of P-type and T-type currents was 95 ± 7% of the total calcium current determined by Mg2+ replacement.
Figure 10.
Ionic currents flowing during the interspike intervals in spontaneous bursts. Indicated values are averaged over a 1.4 msec window (gray box) starting 4.8 msec after the post-spike trough. All currents, including P-type and T-type calcium currents, were recorded in a single cell. Sodium currents are as recorded in reduced NaCl.
Figure 11.
Ionic currents flowing during the first and second intervals of spontaneous bursts of Purkinje neurons. Sodium current densities shown were scaled up by a factor of 3 from those measured in 50 m
m
sodium. Values are averaged over a 1.4 msec window starting 4.8 msec after the post-spike trough. For sodium current and potassium currents, n = 5 cells. For P-type and T-type currents, n = 18 cells. *p < 0.01; paired t test.
Similar articles
- Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons.
Raman IM, Bean BP. Raman IM, et al. J Neurosci. 1999 Mar 1;19(5):1663-74. doi: 10.1523/JNEUROSCI.19-05-01663.1999. J Neurosci. 1999. PMID: 10024353 Free PMC article. - Robustness of burst firing in dissociated purkinje neurons with acute or long-term reductions in sodium conductance.
Swensen AM, Bean BP. Swensen AM, et al. J Neurosci. 2005 Apr 6;25(14):3509-20. doi: 10.1523/JNEUROSCI.3929-04.2005. J Neurosci. 2005. PMID: 15814781 Free PMC article. - Dendritic control of spontaneous bursting in cerebellar Purkinje cells.
Womack MD, Khodakhah K. Womack MD, et al. J Neurosci. 2004 Apr 7;24(14):3511-21. doi: 10.1523/JNEUROSCI.0290-04.2004. J Neurosci. 2004. PMID: 15071098 Free PMC article. - Matching molecules to function: neuronal Ca2+-activated K+ channels and afterhyperpolarizations.
Stocker M, Hirzel K, D'hoedt D, Pedarzani P. Stocker M, et al. Toxicon. 2004 Jun 15;43(8):933-49. doi: 10.1016/j.toxicon.2003.12.009. Toxicon. 2004. PMID: 15208027 Review. - A five-conductance model of the action potential in the rat sympathetic neurone.
Belluzzi O, Sacchi O. Belluzzi O, et al. Prog Biophys Mol Biol. 1991;55(1):1-30. doi: 10.1016/0079-6107(91)90009-h. Prog Biophys Mol Biol. 1991. PMID: 2057576 Review.
Cited by
- Action potential processing in a detailed Purkinje cell model reveals a critical role for axonal compartmentalization.
Masoli S, Solinas S, D'Angelo E. Masoli S, et al. Front Cell Neurosci. 2015 Feb 24;9:47. doi: 10.3389/fncel.2015.00047. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25759640 Free PMC article. - A sodium afterdepolarization in rat superior colliculus neurons and its contribution to population activity.
Ghitani N, Bayguinov PO, Basso MA, Jackson MB. Ghitani N, et al. J Neurophysiol. 2016 Jul 1;116(1):191-200. doi: 10.1152/jn.01138.2015. Epub 2016 Apr 13. J Neurophysiol. 2016. PMID: 27075543 Free PMC article. - Ca²⁺-dependent regulation of Ca²⁺ currents in rat primary afferent neurons: role of CaMKII and the effect of injury.
Tang Q, Bangaru ML, Kostic S, Pan B, Wu HE, Koopmeiners AS, Yu H, Fischer GJ, McCallum JB, Kwok WM, Hudmon A, Hogan QH. Tang Q, et al. J Neurosci. 2012 Aug 22;32(34):11737-49. doi: 10.1523/JNEUROSCI.0983-12.2012. J Neurosci. 2012. PMID: 22915116 Free PMC article. - Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones.
Rush AM, Dib-Hajj SD, Waxman SG. Rush AM, et al. J Physiol. 2005 May 1;564(Pt 3):803-15. doi: 10.1113/jphysiol.2005.083089. Epub 2005 Mar 10. J Physiol. 2005. PMID: 15760941 Free PMC article. - A cytoplasmic Slo3 isoform is expressed in somatic tissues.
Chávez JC, Vicens A, Wrighton DC, Andrade-López K, Beltrán C, Gutiérrez RM, Lippiat JD, Treviño CL. Chávez JC, et al. Mol Biol Rep. 2019 Oct;46(5):5561-5567. doi: 10.1007/s11033-019-04943-z. Epub 2019 Jul 3. Mol Biol Rep. 2019. PMID: 31270758
References
- Callaway JC, Ross WN ( 1997) Spatial distribution of synaptically activated sodium concentration changes in cerebellar Purkinje neurons. J Neurophysiol 77: 145-152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources