Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo - PubMed (original) (raw)
Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo
Steve D Swain et al. Infect Immun. 2003 Nov.
Abstract
Host defense against the opportunistic pathogen Pneumocystis carinii requires functional interactions of many cell types. Alveolar macrophages are presumed to be a vital host cell in the clearance of P. carinii, and the mechanisms of this interaction have come under scrutiny. The macrophage mannose receptor is believed to play an important role as a receptor involved in the binding and phagocytosis of P. carinii. Although there is in vitro evidence for this interaction, the in vivo role of this receptor in P. carinii clearance in unclear. Using a mouse model in which the mannose receptor has been deleted, we found that the absence of this receptor is not sufficient to allow infection by P. carinii in otherwise immunocompetent mice. Furthermore, when mice were rendered susceptible to P. carinii by CD4(+) depletion, mannose receptor knockout mice (MR-KO) had pathogen loads equal to those of wild-type mice. However, the MR-KO mice exhibited a greater influx of phagocytes into the alveoli during infection. This was accompanied by increased pulmonary pathology in the MR-KO mice, as well as greater accumulation of glycoproteins in the alveoli (glycoproteins, including harmful hydrolytic enzymes, are normally cleared by the mannose receptor). We also found that the surface expression of the mannose receptor is not downregulated during P. carinii infection in wild-type mice. Our findings suggest that while the macrophage mannose receptor may be important in the recognition of P. carinii, in vivo, this mechanism may be redundant, and the absence of this receptor may be compensated for.
Figures
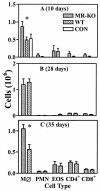
FIG. 1.
Inflammatory cells in the BAL of _P. carinii_-inoculated MR-KO mice (grey bar), wild type (WT) mice (slashed bar), and uninfected wild-type (CON, panel A only) (open bar) mice. (A) Ten days after i.t. inoculation; (B) 28 days after i.t. inoculation; (C) after 35 days of continuous exposure to P. carinii via cohabitation with previously infected SCID mice. Cell types are macrophages (MØ), neutrophils (PMN), CD4+ lymphocytes (CD4+), and CD8+ lymphocytes (CD8+). *, MR-KO mice are significantly different from WT mice (P ≤ 0.05). Values are means ± standard error of the mean; n = 4 to 6.
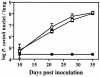
FIG. 2.
Pneumocystis levels in the lungs of MR-KO and wild-type mice that have been immunocompromised by the depletion of CD4+ lymphocytes. Mice were inoculated i.t. with 107 P. carinii nuclei. At the indicated days, the pulmonary pathogen load was determined by visual counts of P. carinii nuclei from lung homogenate samples. □, MR-KO; ○, wild type; ▪, wild-type uninoculated control. Values are means ± standard deviation; n = 5 except for MR-KO 10-day (n = 6), and MR-KO 21-day (n = 4) results.
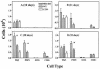
FIG. 3.
Inflammatory cells in the BAL of CD4+ lymphocyte-depleted and _P. carinii_-inoculated MR-KO (grey bar) mice, wild-type (WT) mice (slashed bar), and nondepleted, uninfected wild-type control (CON) mice (open bar). (A) Ten days after i.t. inoculation; (B) 21 days after inoculation; (C) 28 days after inoculation; (D) 35 days after inoculation. Cell types are macrophages (MØ), neutrophils (PMN), and CD8+ lymphocytes (CD8+). *, MR-KO mice are significantly different from WT mice (P ≤ 0.05). Values are means ± standard of the mean; n = 5.
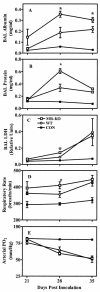
FIG.4.
Respiratory pathology in CD4+ lymphocyte-depleted and _P. carinii_-infected MR-KO and wild-type (WT) mice. (A) Albumin concentration in 5-ml lavage samples. (B) Total protein concentration in lavage samples. (C) LDH concentration in 5-ml lavage samples. (D) Rate of respiration of mice prior to tissue sampling. (E) Partial pressure of O2 in blood sampled from the tail artery immediately prior to sacrifice. □, MR-KO; ○, WT; ▪, wild-type uninoculated control. Values are means ± standard deviation; n = 4 to 6. x axis refers to number of days since inoculation with P. carinii. *, MR-KO mice are significantly different from WT mice (P ≤ 0.05).
FIG. 5.
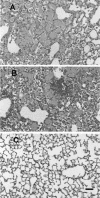
Pulmonary histology in CD4+ lymphocyte-depleted and _P. carinii_-infected MR-KO and wild type (WT) mice. Shown are hematoxylin- and eosin-stained paraffin sections from formalin-fixed lungs of MR-KO (A), WT (B), and control untreated (C) mice. Samples are from mice 35 days post-infection. Both MR-KO (A) and WT (B) lungs exhibited widespread alveolar filling with P. carinii organisms and mixed inflammatory cell infiltrate. Black bar in panel C, 100 μM. The three pictures are of equal magnification.
FIG. 6.
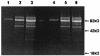
Total glycoproteins in BAL from CD4+-depleted and _P. carinii_-infected MR-KO and wild-type mice. Proteins in BAL samples were resolved on a 4 to 20% SDS polyacrylamide gel, periodate oxidized, and stained with a fluorescent probe. Each lane is sample pooled from three mice in each group. Lanes 1, 2, and 3 are, respectively, control (uninoculated wild-type) mice, infected wild-type mice, and infected MR-KO mice at 28 days postinoculation. Lanes 4, 5, and 6 are control mice, infected wild-type mice, and infected MR-KO mice at 35 days postinoculation.
FIG. 7.
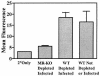
Binding of mannosylated BSA to alveolar macrophages. BAL macrophages were incubated with mannose-BSA-biotin in the presence of calcium and then washed and incubated with streptavidin-APC. Geometric mean fluorescence of the cells was determined by flow cytometry. Cells are from mice that were CD4+ lymphocyte depleted and P. carinii infected for 28 days: MR-KO is infected MR-KO mice, and WT is infected wild-type mice. Control cells are from wild-type mice that have not been depleted or infected. 2°Only refers to cells from wild-type-infected mice that were incubated with streptavidin-APC but not mannose BSA. Bars represent mean ± standard error of the mean; n = 5. *, MR-KO mice are significantly different from WT mice (P ≤ 0.01).
Similar articles
- Pneumocystis carinii enhances soluble mannose receptor production by macrophages.
Fraser IP, Takahashi K, Koziel H, Fardin B, Harmsen A, Ezekowitz RA. Fraser IP, et al. Microbes Infect. 2000 Sep;2(11):1305-10. doi: 10.1016/s1286-4579(00)01283-1. Microbes Infect. 2000. PMID: 11018446 - Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation.
Koziel H, Eichbaum Q, Kruskal BA, Pinkston P, Rogers RA, Armstrong MY, Richards FF, Rose RM, Ezekowitz RA. Koziel H, et al. J Clin Invest. 1998 Oct 1;102(7):1332-44. doi: 10.1172/JCI560. J Clin Invest. 1998. PMID: 9769325 Free PMC article. - Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor.
Ezekowitz RA, Williams DJ, Koziel H, Armstrong MY, Warner A, Richards FF, Rose RM. Ezekowitz RA, et al. Nature. 1991 May 9;351(6322):155-8. doi: 10.1038/351155a0. Nature. 1991. PMID: 1903183 - From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor.
Allavena P, Chieppa M, Monti P, Piemonti L. Allavena P, et al. Crit Rev Immunol. 2004;24(3):179-92. doi: 10.1615/critrevimmunol.v24.i3.20. Crit Rev Immunol. 2004. PMID: 15482253 Review. - The mannose receptor in the brain.
Régnier-Vigouroux A. Régnier-Vigouroux A. Int Rev Cytol. 2003;226:321-42. doi: 10.1016/s0074-7696(03)01006-4. Int Rev Cytol. 2003. PMID: 12921240 Review.
Cited by
- Trichuris suis induces human non-classical patrolling monocytes via the mannose receptor and PKC: implications for multiple sclerosis.
Kooij G, Braster R, Koning JJ, Laan LC, van Vliet SJ, Los T, Eveleens AM, van der Pol SM, Förster-Waldl E, Boztug K, Belot A, Szilagyi K, van den Berg TK, van Buul JD, van Egmond M, de Vries HE, Cummings RD, Dijkstra CD, van Die I. Kooij G, et al. Acta Neuropathol Commun. 2015 Jul 25;3:45. doi: 10.1186/s40478-015-0223-1. Acta Neuropathol Commun. 2015. PMID: 26205402 Free PMC article. - C-type lectins on macrophages participate in the immunomodulatory response to Fasciola hepatica products.
Guasconi L, Serradell MC, Garro AP, Iacobelli L, Masih DT. Guasconi L, et al. Immunology. 2011 Jul;133(3):386-96. doi: 10.1111/j.1365-2567.2011.03449.x. Epub 2011 May 20. Immunology. 2011. PMID: 21595685 Free PMC article. - Expression of the mannose receptor CD206 in HIV and SIV encephalitis: a phenotypic switch of brain perivascular macrophages with virus infection.
Holder GE, McGary CM, Johnson EM, Zheng R, John VT, Sugimoto C, Kuroda MJ, Kim WK. Holder GE, et al. J Neuroimmune Pharmacol. 2014 Dec;9(5):716-26. doi: 10.1007/s11481-014-9564-y. Epub 2014 Aug 22. J Neuroimmune Pharmacol. 2014. PMID: 25146376 Free PMC article. - Fungal Strategies to Evade the Host Immune Recognition.
Hernández-Chávez MJ, Pérez-García LA, Niño-Vega GA, Mora-Montes HM. Hernández-Chávez MJ, et al. J Fungi (Basel). 2017 Sep 23;3(4):51. doi: 10.3390/jof3040051. J Fungi (Basel). 2017. PMID: 29371567 Free PMC article. Review. - Colonization by Pneumocystis jirovecii and its role in disease.
Morris A, Norris KA. Morris A, et al. Clin Microbiol Rev. 2012 Apr;25(2):297-317. doi: 10.1128/CMR.00013-12. Clin Microbiol Rev. 2012. PMID: 22491773 Free PMC article. Review.
References
- Beck, J. M., and A. G. Harmsen. 1998. Lymphocytes in host defense against Pneumocystis carinii. Semin. Respir. Infect. 13:330-338. - PubMed
- Beck, J. M., R. L. Newbury, B. E. Palmer, M. L. Warnock, P. K. Byrd, and H. B. Kaltreider. 1996. Role of CD8+ lymphocytes in host defense against Pneumocystis carinii in mice. J. Lab. Clin. Med. 128:477-487. - PubMed
- Beck, J. M., M. L. Warnock, J. L. Curtis, M. J. Sniezek, S. M. Arraj-Peffer, H. B. Kaltreider, and J. E. Shellito. 1991. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am. J. Respir. Cell Mol. Biol. 5:186-197. - PubMed
- Beck, J. M., M. L. Warnock, H. B. Kaltreider, and J. E. Shellito. 1993. Host defenses against Pneumocystis carinii in mice selectively depleted of CD4+ lymphocytes. Chest 103:116S-118S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials