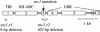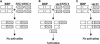A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1 - PubMed (original) (raw)
. 2003 Nov;15(11):2636-46.
doi: 10.1105/tpc.015842. Epub 2003 Oct 23.
Affiliations
- PMID: 14576290
- PMCID: PMC280567
- DOI: 10.1105/tpc.015842
A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1
Yuelin Zhang et al. Plant Cell. 2003 Nov.
Abstract
Plants have evolved sophisticated defense mechanisms against pathogen infections, during which resistance (R) genes play central roles in recognizing pathogens and initiating defense cascades. Most of the cloned R genes share two common domains: the central domain, which encodes a nucleotide binding adaptor shared by APAF-1, certain R proteins, and CED-4 (NB-ARC), plus a C-terminal region that encodes Leu-rich repeats (LRR). In Arabidopsis, a dominant mutant, suppressor of npr1-1, constitutive 1 (snc1), was identified previously that constitutively expresses pathogenesis-related (PR) genes and resistance against both Pseudomonas syringae pv maculicola ES4326 and Peronospora parasitica Noco2. The snc1 mutation was mapped to the RPP4 cluster. In snc1, one of the TIR-NB-LRR-type R genes contains a point mutation that results in a single amino acid change from Glu to Lys in the region between NB-ARC and LRR. Deletions of this R gene in snc1 reverted the plants to wild-type morphology and completely abolished constitutive PR gene expression and disease resistance. The constitutive activation of the defense responses was not the result of the overexpression of the R gene, because its expression level was not altered in snc1. Our data suggest that the point mutation in snc1 renders the R gene constitutively active without interaction with pathogens. To analyze signal transduction pathways downstream of snc1, epistasis analyses between snc1 and pad4-1 or eds5-3 were performed. Although the resistance signaling in snc1 was fully dependent on PAD4, it was only partially affected by blocking salicylic acid (SA) synthesis, suggesting that snc1 activates both SA-dependent and SA-independent resistance pathways.
Figures
Figure 1.
Gene Structure of SNC1 (At4g16890). Exons (rectangles) and introns (lines) are drawn in proportion to their lengths. Exons encoding the TIR (exon 1), NB-ARC (exon 2), and LRR (exons 4 to 7) domains are indicated. The position of the G-to-A mutation in snc1 is indicated by the arrow. The two revertant mutations (snc1-r1 and snc1-r2) are shown as triangles.
Figure 2.
Sequence Analysis of SNC1 and the snc1 Mutation. (A) Phylogenetic relationship of SNC1 and other TIR-NBS-LRR–type R proteins. The neighbor-joining tree was constructed with BIONJ (Gascuel, 1997) using proteins aligned with CLUSTAL W. Distances were calculated with TREE-PUZZLE 5.0 (Strimmer and von Haeseler, 1996) using the WAG substitution matrix. (B) Amino acid sequence comparison of SNC1 exon 3 with other TIR-NBS-LRR proteins. The alignment was performed using CLUSTAL W. The predicted Glu-to-Lys (E→K) change in snc1 is highlighted (asterisk). Colons indicate high amino acid identity, and dots indicate low identity. RPP4, Resistance to Peronospora parasitica 4 (van der Biezen et al., 2002); RPP5, Resistance to P. parasitica 5 (Parker et al., 1997); RPP1-WsA to RPP1-WsC, Resistance to P. parasitica 1, Arabidopsis thaliana accession Wassilewskija A to C (Botella et al., 1998); SSI4, suppressor of salicylic acid insensitivity of npr1-5, 4 (Shirano et al., 2002); N, Nicotiana glutinosa virus resistance gene (Whitham et al., 1994); RPS4, Resistance to Pseudomonas syringae 4 (Gassmann et al., 1999); L6, Linum usitatissimum rust resistance gene (Lawrence et al., 1995); RRS1-R, Resistance to Ralstonia solanacearum 1-recessive (Deslandes et al., 2002). (C) Locations of the snc1 mutation and amino acid changes in confirmed gain-of-function R protein variants that cause constitutive HR.
Figure 3.
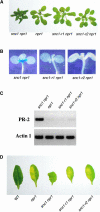
Phenotypic Analysis of snc1 Revertants. (A) Morphological phenotypes of the snc1 revertants. All plants were grown in parallel on soil and photographed when they were 4 weeks old. (B) Suppression of _snc1_-induced _pBGL2_-GUS reporter gene expression in the revertants. Three-week-old seedlings grown on MS medium were stained for GUS activity from _pBGL2_-GUS. (C) PR-2 (BGL2) gene expression in snc1 revertants. RNAs were prepared from 20-day-old plants grown on MS medium and reverse transcribed to obtain total cDNA. The cDNA samples were normalized by real-time PCR using an Actin1 probe. PR-2 and Actin1 in different mutant plants then were amplified by 35 cycles of PCR using equal amounts of total cDNA, and the products were analyzed by agarose gel electrophoresis and ethidium bromide staining. (D) Enhanced disease susceptibility of snc1 revertants to P.s.m. ES4326. Leaves from 4-week-old plants were infiltrated with a P.s.m. ES4326 suspension in 10 mM MgCl2 (OD600 = 0.0001). Photographs were taken of representative leaves 4 days after infection. WT, wild type.
Figure 4.
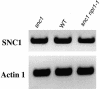
Analysis of SNC1 Expression Levels in Wild-Type and Mutant Plants. RNAs were prepared from 20-day-old plants grown on MS medium and reverse transcribed to obtain cDNA. The cDNA samples were first normalized by real-time PCR using an Actin1 probe. SNC1 and Actin1 in wild-type (WT) and mutant plants then were amplified by 30 cycles of PCR using equal amounts of total cDNA, and the products were analyzed by agarose gel electrophoresis and ethidium bromide staining.
Figure 5.
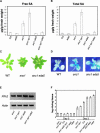
Analysis of snc1 pad4-1. (A) Morphology of snc1 pad4-1 plants. The photograph shows 4-week-old plants grown on soil. (B) Suppression of constitutive _pBGL2_-GUS reporter gene expression in snc1 by pad4-1. Twenty-day-old seedlings grown on MS medium were stained for GUS activity. (C) Enhanced susceptibility of the snc1 pad4-1 double mutant to P.s.m. ES4326. The leaves of 4-week-old soil-grown plants were infiltrated with a suspension of P.s.m. ES4326 in 10 mM MgCl2 (OD600 = 0.00005). The photograph was taken 3 days after infection. At this dose, wild-type (WT) Arabidopsis normally is resistant to the bacterium. (D) Susceptibility of snc1 pad4-1 to P. parasitica Noco2. Two-week-old seedlings were sprayed with Noco2 spores at a conidiospore suspension concentration of 5 × 103 spores per milliliter of water. The infection was rated as follows on 20 plants at 6 days after infection by counting the number of conidiophores per infected leaf: 0, no conidiophores on the plants; 1, no more than 5 conidiophores per infected leaf; 2, 6 to 20 conidiophores on a few of the infected leaves; 3, 6 to 20 conidiophores on most of the infected leaves; 4, 5 or more conidiophores on all infected leaves; 5, 20 or more conidiophores on all infected leaves. Col, Columbia wild type.
Figure 6.
Epistasis Analysis of snc1 and eds5-3. (A) and (B) Free SA (A) and total SA (B) levels in the mutants. Leaf tissue was harvested 4 weeks after germination and used for SA extraction. Each treatment had four replicates. Col, Columbia wild type. (C) Morphology of snc1 eds5 compared with snc1 and the wild type (WT). (D) GUS staining of the _pBGL2_-GUS reporter gene in snc1 and snc1 eds5. Staining was performed on 20-day-old plants grown on MS medium. (E) PR-2 expression in the mutants. RT-PCR was used to analyze the expression of PR-2 as described for Figure 3. (F) Bacterial growth of P.s.m. ES4326 in the mutants. Two leaves of each plant were infiltrated with the bacteria (OD600 = 0.00005). Leaf discs within the inoculated areas were taken after 0 and 3 days of infiltration. Four replicates were taken for each treatment. Error bars represent 95% confidence limits of log-transformed data. cfu, colony-forming units.
Figure 7.
Model for the Activation of SNC1 by the snc1 Mutation. (A) In wild-type plants, SNC1 is sequestered by the proposed negative regulator RBP and is inactive. (B) In snc1/SNC1 plants, some snc1 protein dissociates from its negative regulator and activates downstream defense pathways. (C) In heterozygous revertant snc1/− plants, all snc1 protein is sequestered as a result of the excess amount of the negative regulator.
Figure 8.
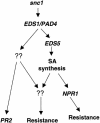
Model for Pathways Activated in snc1 Plants.
Similar articles
- Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance.
Li X, Clarke JD, Zhang Y, Dong X. Li X, et al. Mol Plant Microbe Interact. 2001 Oct;14(10):1131-9. doi: 10.1094/MPMI.2001.14.10.1131. Mol Plant Microbe Interact. 2001. PMID: 11605952 - A putative nucleoporin 96 Is required for both basal defense and constitutive resistance responses mediated by suppressor of npr1-1,constitutive 1.
Zhang Y, Li X. Zhang Y, et al. Plant Cell. 2005 Apr;17(4):1306-16. doi: 10.1105/tpc.104.029926. Epub 2005 Mar 16. Plant Cell. 2005. PMID: 15772285 Free PMC article. - Ethylene and jasmonic acid signaling affect the NPR1-independent expression of defense genes without impacting resistance to Pseudomonas syringae and Peronospora parasitica in the Arabidopsis ssi1 mutant.
Nandi A, Kachroo P, Fukushige H, Hildebrand DF, Klessig DF, Shah J. Nandi A, et al. Mol Plant Microbe Interact. 2003 Jul;16(7):588-99. doi: 10.1094/MPMI.2003.16.7.588. Mol Plant Microbe Interact. 2003. PMID: 12848424 - Complex regulation of an R gene SNC1 revealed by auto-immune mutants.
Gou M, Hua J. Gou M, et al. Plant Signal Behav. 2012 Feb;7(2):213-6. doi: 10.4161/psb.18884. Epub 2012 Feb 1. Plant Signal Behav. 2012. PMID: 22415045 Free PMC article. Review. - Closing another gap in the plant SAR puzzle.
Somssich IE. Somssich IE. Cell. 2003 Jun 27;113(7):815-6. doi: 10.1016/s0092-8674(03)00473-2. Cell. 2003. PMID: 12837238 Review.
Cited by
- Isolation and characterization of the plant immune-priming compounds Imprimatin B3 and -B4, potentiators of disease resistance in Arabidopsis thaliana.
Noutoshi Y, Okazaki M, Shirasu K. Noutoshi Y, et al. Plant Signal Behav. 2012 Dec;7(12):1526-8. doi: 10.4161/psb.22138. Epub 2012 Oct 16. Plant Signal Behav. 2012. PMID: 23073018 Free PMC article. - Mutations in nuclear pore complex promote osmotolerance in Arabidopsis by suppressing the nuclear translocation of ACQOS and its osmotically induced immunity.
Mori K, Murakoshi Y, Tamura M, Kunitake S, Nishimura K, Ariga H, Tanaka K, Iuchi S, Yotsui I, Sakata Y, Taji T. Mori K, et al. Front Plant Sci. 2024 Jan 22;15:1304366. doi: 10.3389/fpls.2024.1304366. eCollection 2024. Front Plant Sci. 2024. PMID: 38318497 Free PMC article. - The ubiquitin system affects agronomic plant traits.
Linden KJ, Callis J. Linden KJ, et al. J Biol Chem. 2020 Oct 2;295(40):13940-13955. doi: 10.1074/jbc.REV120.011303. Epub 2020 Aug 12. J Biol Chem. 2020. PMID: 32796036 Free PMC article. Review. - Regulation of plant innate immunity by three proteins in a complex conserved across the plant and animal kingdoms.
Palma K, Zhao Q, Cheng YT, Bi D, Monaghan J, Cheng W, Zhang Y, Li X. Palma K, et al. Genes Dev. 2007 Jun 15;21(12):1484-93. doi: 10.1101/gad.1559607. Genes Dev. 2007. PMID: 17575050 Free PMC article. - Overexpression of NDR1 leads to pathogen resistance at elevated temperatures.
Samaradivakara SP, Chen H, Lu YJ, Li P, Kim Y, Tsuda K, Mine A, Day B. Samaradivakara SP, et al. New Phytol. 2022 Aug;235(3):1146-1162. doi: 10.1111/nph.18190. Epub 2022 May 21. New Phytol. 2022. PMID: 35488494 Free PMC article.
References
- Axtell, M.J., McNellis, T.W., Mudgett, M.B., Hsu, C.S., and Staskawicz, B.J. (2001). Mutational analysis of the Arabidopsis RPS2 disease resistance gene and the corresponding Pseudomonas syringae avrRpt2 avirulence gene. Mol. Plant-Microbe Interact. 14, 181–188. - PubMed
- Axtell, M.J., and Staskawicz, B.J. (2003). Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112, 369–377. - PubMed
- Baker, B., Zambryski, P., Staskawicz, B., and Dinesh-Kumar, S.P. (1997). Signaling in plant-microbe interactions. Science 276, 726–733. - PubMed
- Bendahmane, A., Farnham, G., Moffett, P., and Baulcombe, D.C. (2002). Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. Plant J. 32, 195–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous