Spontaneous changes in mitochondrial membrane potential in single isolated brain mitochondria - PubMed (original) (raw)
Spontaneous changes in mitochondrial membrane potential in single isolated brain mitochondria
Olga Vergun et al. Biophys J. 2003 Nov.
Abstract
In this study we measured DeltaPsim in single isolated brain mitochondria using rhodamine 123. Mitochondria were attached to coverslips and superfused with K(+)-based HEPES-buffer medium supplemented with malate and glutamate. In approximately 70% of energized mitochondria we observed large amplitude spontaneous fluctuations in DeltaPsim with a time course comparable to that observed previously in mitochondria of intact cells. The other 30% of mitochondria maintained a stable DeltaPsim. Some of the "stable" mitochondria began to fluctuate spontaneously during the recording period. However, none of the initially fluctuating mitochondria became stable. Upon the removal of substrates from the medium or application of small amounts of Ca(2+), rhodamine 123 fluorescence rapidly dropped to background values in fluctuating mitochondria, while nonfluctuating mitochondria depolarized with a delay and often began to fluctuate before complete depolarization. The changes in DeltaPsim were not connected to oxidant production since reducing illumination or the addition of antioxidants had no effect on DeltaPsim. Fluctuating mitochondria did not lose calcein, nor was there any effect of cyclosporin A on DeltaPsim, which ruled out a contribution of permeability transition. We conclude that the fluctuations in DeltaPsim reflect an intermediate, unstable state of mitochondria that may lead to or reflect mitochondrial dysfunction.
Figures
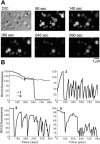
FIGURE 1
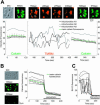
Examples of characteristic changes in Rh123 fluorescence in single mitochondria. (A) Representative DIC image and fluorescent images taken at different time points illustrating spontaneous ΔΨm fluctuations. (B) The plots show different patterns of spontaneous changes in Rh123 fluorescence in five individual mitochondria from panel A. Mitochondria 1 and 2 would be considered nonfluctuating, whereas 3–5 show various characteristics of individual mitochondria with fluctuating ΔΨm.
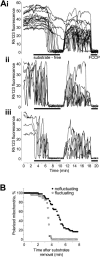
FIGURE 2
Time course of the mitochondrial depolarization in the response to removal of substrates and addition of Ca2+. (A) Changes in Rh123 fluorescence in nonfluctuating (i), fluctuating (ii), and initially nonfluctuating (iii) individual mitochondria. (B) Summarized data of the single experiment presented in A, showing the rate of depolarization in fluctuating compared to nonfluctuating mitochondria. The time of the beginning of the application of substrate-free medium was defined as 0. The percentage of polarized mitochondria was calculated from 150 individual mitochondria; mitochondria that had an Rh123 fluorescence level less than four units were determined as depolarized. These results are representative of four additional experiments.
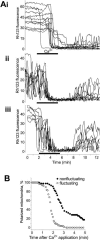
FIGURE 3
The effects of Ca2+ on ΔΨm in individual mitochondria. A total of 35 _μ_M Ca2+ was added to the normal 20 _μ_M EDTA-containing superfusate, yielding a free calcium concentration of ∼15_μ_M. (A) Each panel shows separately nonfluctuating (i), fluctuating (ii), and initially nonfluctuating (iii) individual mitochondria. (B) Percentage of mitochondria remaining polarized shown as a function of time after Ca2+ application. Traces from individual mitochondria were segregated into fluctuating and nonfluctuating based on the Rh123 signal before calcium application. The time of the beginning of the application of substrate-free medium was defined as 0. These curves represent the mean of 200 individual mitochondria from the same field, and are representative of five additional experiments.
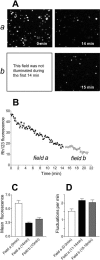
FIGURE 4
Effect of illumination on ΔΨm in single isolated mitochondria. (A) Images of Rh123 fluorescence in the two different fields of the coverslip with the attached mitochondria. The mitochondria in panels a were subjected to the normal illumination, whereas the mitochondria in panel b were illuminated only at the end of the experiment. (B) The average Rh123 fluorescence of 150 individual mitochondria from each field shown in the previous panel. Panels A and B are from a single experiment that was repeated four additional times with similar results. (C and D) Mean Rh123 fluorescence and the frequency of the fluctuations, respectively, were not significantly different in the mitochondria from field a and b when they were measured at the closed time points. The data in C and D represent the mean ± SE of five experiments.
FIGURE 5
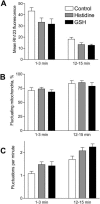
Effect of antioxidants on ΔΨm. A scavenger of singlet oxygen histidine (300 _μ_M) or glutathione (GSH, 5 mM) did not have any effect on ΔΨm. All parameters (mean Rh123 fluorescence, percentage of blinking mitochondria, and frequency of fluctuations) were measured at the beginning (1–3 min) and at the end (12–15 min) of the experiment. The antioxidants were added to the perfusion medium for 2 min before the start of the recording and were present for the entire experiment. The data represent the mean ± SE of five experiments.
FIGURE 6
The measurement of calcein fluorescence in single mitochondria. (A) The calcein fluorescence and ΔΨm (TMRM) were measured in the same mitochondria (see explanation in the text). The series of images at the top represent DIC and fluorescent images taken at the different time points indicated. The images show the fluorescence of calcein (green images) and TMRM (red images). The traces illustrate individual mitochondria and mean calcein signal (± SE, n = 100 mitochondria). These data are representative of five additional experiments. (B) Left panel, DIC image and fluorescence images showing the loss of calcein fluorescence in the presence of 40 _μ_g/ml alamethicin; right panel, the traces obtained from individual mitochondria and mean calcein signal (± SE, n = 50 mitochondria). The data are representative of three additional experiments. (C) Alamethicin-induced depolarization of ΔΨm measured by Rh123. The concentration of alamethicin was 40 _μ_g/ml. Each trace corresponds to an individual mitochondrion.
FIGURE 7
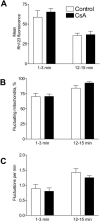
Effect of cyclosporin A (CsA) on ΔΨm. The histograms show a mean Rh123 fluorescence (A), percentage of fluctuating mitochondria (B), and frequency of fluctuations (C) in single mitochondria in control and 1 _μ_M CsA-containing medium. Each histogram represents the mean (± SE) of five experiments, and 150 mitochondria were analyzed in each experiment. None of the relevant differences reached statistical significance (P > 0.05).
Similar articles
- Fluctuations in mitochondrial membrane potential in single isolated brain mitochondria: modulation by adenine nucleotides and Ca2+.
Vergun O, Reynolds IJ. Vergun O, et al. Biophys J. 2004 Nov;87(5):3585-93. doi: 10.1529/biophysj.104.042671. Epub 2004 Aug 17. Biophys J. 2004. PMID: 15315954 Free PMC article. - Role of oxidative stress in the ammonia-induced mitochondrial permeability transition in cultured astrocytes.
Rama Rao KV, Jayakumar AR, Norenberg MD. Rama Rao KV, et al. Neurochem Int. 2005 Jul;47(1-2):31-8. doi: 10.1016/j.neuint.2005.04.004. Neurochem Int. 2005. PMID: 15908047 - Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore.
Hüser J, Blatter LA. Hüser J, et al. Biochem J. 1999 Oct 15;343 Pt 2(Pt 2):311-7. Biochem J. 1999. PMID: 10510294 Free PMC article. - Repetitive transient depolarizations of the inner mitochondrial membrane induced by proton pumping.
Hattori T, Watanabe K, Uechi Y, Yoshioka H, Ohta Y. Hattori T, et al. Biophys J. 2005 Mar;88(3):2340-9. doi: 10.1529/biophysj.104.041483. Epub 2005 Jan 14. Biophys J. 2005. PMID: 15653749 Free PMC article. - Advances in the analysis of single mitochondria.
Fuller KM, Arriaga EA. Fuller KM, et al. Curr Opin Biotechnol. 2003 Feb;14(1):35-41. doi: 10.1016/s0958-1669(02)00008-3. Curr Opin Biotechnol. 2003. PMID: 12566000 Review.
Cited by
- Cristae remodeling causes acidification detected by integrated graphene sensor during mitochondrial outer membrane permeabilization.
Pham TD, Pham PQ, Li J, Letai AG, Wallace DC, Burke PJ. Pham TD, et al. Sci Rep. 2016 Oct 27;6:35907. doi: 10.1038/srep35907. Sci Rep. 2016. PMID: 27786282 Free PMC article. - Mitochondrial calcium ion and membrane potential transients follow the pattern of epileptiform discharges in hippocampal slice cultures.
Kovács R, Kardos J, Heinemann U, Kann O. Kovács R, et al. J Neurosci. 2005 Apr 27;25(17):4260-9. doi: 10.1523/JNEUROSCI.4000-04.2005. J Neurosci. 2005. PMID: 15858052 Free PMC article. - Mitochondrial oscillations in physiology and pathophysiology.
Aon MA, Cortassa S, O'Rourke B. Aon MA, et al. Adv Exp Med Biol. 2008;641:98-117. doi: 10.1007/978-0-387-09794-7_8. Adv Exp Med Biol. 2008. PMID: 18783175 Free PMC article. Review. - Crosstalk signaling between mitochondrial Ca2+ and ROS.
Feissner RF, Skalska J, Gaum WE, Sheu SS. Feissner RF, et al. Front Biosci (Landmark Ed). 2009 Jan 1;14(4):1197-218. doi: 10.2741/3303. Front Biosci (Landmark Ed). 2009. PMID: 19273125 Free PMC article. Review. - Fluctuations in mitochondrial membrane potential in single isolated brain mitochondria: modulation by adenine nucleotides and Ca2+.
Vergun O, Reynolds IJ. Vergun O, et al. Biophys J. 2004 Nov;87(5):3585-93. doi: 10.1529/biophysj.104.042671. Epub 2004 Aug 17. Biophys J. 2004. PMID: 15315954 Free PMC article.
References
- Andreyev, A. Y., B. Fahy, and G. Fiskum. 1998. Cyctochrome c release from brain mitochondria is independent of the mitochondrial permeability transition. FEBS Lett. 439:373–376. - PubMed
- Andreyev, A., and G. Fiskum. 1999. Calcium induced release of mitochondrial cytochrome c by different mechanisms selective for brain versus liver. Cell Death Differ. 6:825–832. - PubMed
- Belousov, V. V., L. L. Bambrick, A. A. Starkov, D. B. Zorov, V. P. Skulachev, and G. Fiskum. 2001. Oscillations in mitochondrial membrane potential in rat astrocytes in vitro. Abstr. Soc. Neurosci. 27:205–218.
- Berman, S. B., S. C. Watkins, and T. G. Hastings. 2000. Quantitative biochemical and ultrastructural comparison of mitochondrial permeability transition in isolated brain and liver mitochondria: evidence for relative insensitivity of brain mitochondria. Exp. Neurol. 164:415–425. - PubMed
- Bernardi, P. 1999. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 79:1127–1155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous