Atomic force microscopy investigation of human immunodeficiency virus (HIV) and HIV-infected lymphocytes - PubMed (original) (raw)
Atomic force microscopy investigation of human immunodeficiency virus (HIV) and HIV-infected lymphocytes
Y G Kuznetsov et al. J Virol. 2003 Nov.
Abstract
Isolated human immunodeficiency virus (HIV) and HIV-infected human lymphocytes in culture have been imaged for the first time by atomic force microscopy (AFM). Purified virus particles spread on glass substrates are roughly spherical, reasonably uniform, though pleomorphic in appearance, and have diameters of about 120 nm. Similar particles are also seen on infected cell surfaces, but morphologies and sizes are considerably more varied, possibly a reflection of the budding process. The surfaces of HIV particles exhibit "tufts" of protein, presumably gp120, which do not physically resemble spikes. The protein tufts, which number about 100 per particle, have average diameters of about 200 A, but with a large variance. They likely consist of arbitrary associations of small numbers of gp120 monomers on the surface. In examining several hundred virus particles, we found no evidence that the gp120 monomers form threefold symmetric trimers. Although >95% of HIV-infected H9 lymphocytic cells were producing HIV antigens by immunofluorescent assay, most lymphocytes displayed few or no virus on their surfaces, while others were almost covered by a hundred or more viruses, suggesting a dependence on cell cycle or physiology. HIV-infected cells treated with a viral protease inhibitor and their progeny viruses were also imaged by AFM and were indistinguishable from untreated virions. Isolated HIV virions were disrupted by exposure to mild neutral detergents (Tween 20 and CHAPS) at concentrations from 0.25 to 2.0%. Among the products observed were intact virions, the remnants of completely degraded virions, and partially disrupted particles that lacked sectors of surface proteins as well as virions that were split or broken open to reveal their empty interiors. Capsids containing nucleic acid were not seen, suggesting that the capsids were even more fragile than the envelope and were totally degraded and lost. From these images, a good estimate of the thickness of the envelope protein-membrane-matrix protein outer shell of the virion was obtained. Treatment with even low concentrations (<0.1%) of sodium dodecyl sulfate completely destroyed all virions but produced many interesting products, including aggregates of viral proteins with strands of nucleic acid.
Figures
FIG. 1.
Isolated HIV particles. AFM images of particles obtained by centrifugation of the culture medium from an HIV-infected cultured human lymphocytic cell line are shown. The resuspended virions were spread on poly-
l
-lysine-coated glass coverslips, fixed with 0.1% glutaraldehyde and 1.0% osmium tetroxide, and imaged by AFM under ethanol. (a to d) Groups of virus particles adhering to the glass substrate. The tendency to form clusters is likely due to packing of particles as a result of centrifugation. (e and f) Two isolated viruses imaged at high resolution showing the distinctive but arbitrary distribution of protein tufts covering their exterior surfaces. The roughly spherical particles have average heights of 120 nm, although some, as in panel f, are seen to be slightly compressed, probably due to contact with the substrate. The particles appear to be soft and easily deformed from a spherical shape. The images seen here are typical of many such particles found on the substrate.
FIG. 2.
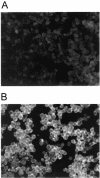
Immunofluorescence assay for H9 (A) and HIV-infected H9 (B) cells. Cells were dried, fixed on glass slides, and incubated first with an IgG fraction from HIV-infected individuals and then with fluorescein-conjugated goat anti-human IgG. Both antigen-positive and antigen-negative cells were observed and counted to determine the percentage of HIV antigen-positive cells. Images were captured with a SPOT Insight charge-coupled device camera.
FIG. 3.
Uninfected human lymphocytes. AFM images of an uninfected human lymphocytic cell line in culture showing the remarkable diversity of their external features are shown. In most cases, there are large smooth surfaces combined with a variety of membranous protrusions, microvilli, and films (a and b). Some cells are almost totally covered with microvilli and membranous structures (c and d [higher resolution]). The cell in panel d exhibits a dense accumulation of microvilli along with a smooth, protruding, dome-like feature. These lymphocytes are the most complex and heterogeneous cells that have so far been visualized by AFM.
FIG. 4.
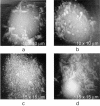
AFM images of HIV-infected H9 cells. (a) AFM image of an HIV-infected H9 cell. On its surface, marked by arrows, emerging HIV particles can be seen. At high resolution, they have the appearances of those particles seen in Fig. 1. The particles are not present on uninfected cells. This cell appears to have, overall, only 5 to 10 HIV particles on its surface, which is about average for the infected cells. Some cells had fewer particles or none at all. Occasionally, cells were observed which appeared to be virtually covered with viral particles (b). (c) AFM image of a cascade of emerging particles on one such cell. (d) An example of microvilli on the surface of an HIV-infected lymphocyte which exhibits a distinctive segmentation. The sizes of the segments, which in some cases are roughly spherical in shape, are in the range of 120 nm, corresponding to the diameters of virus particles.
FIG. 5.
HIV on cell surfaces. Shown is a gallery of AFM images of HIV particles emerging from or attached to the surfaces of human lymphocytes. The complex virion surfaces vary in detail from particle to particle, suggesting that the clusters of envelope proteins arise from more or less arbitrary associations of subunits. Particles have average diameters of 127 nm and exhibit exteriors similar to those in Fig. 1.
FIG. 6.
Detergent-disrupted virions. When HIV virions collected from their media are spread on glass slips and treated with mild detergents, partially degraded or damaged virions are frequent products. (a) Particle with a sector of its surface missing, which leaves a deep pit leading to the interior. Such particles are rarely seen in HIV populations in the absence of detergent treatment. (b to d) More severely disrupted particles. The particle in panel b has a large gaping cavity, and for the one in panel c, the top of the particle appears broken open and exposed. The particle in panel d is completely split open. The view is of the interior surface of the envelope and the cavity once occupied by the capsid. There is also, in all images, a heavy background of fragments, proteins, and viral debris from the disrupted particles.
FIG. 7.
Protein-nucleic acid complexes. HIV virions disrupted by incubation with 0.5% SDS, a strong detergent, are shown. When HIV virions spread on glass are disrupted with a strong detergent, nearly complete degradation occurs and elements of the nucleic acid, almost always associated with proteins and protein aggregates, are a major product.
FIG. 8.
Protease inhibitor-treated cells. (a to d) Infected cells treated with the protease inhibitor nelfinavir. The particles are not perceptibly different in surface character from those on untreated cells. (e and f) Particles collected from the medium of HIV-infected cells treated with the protease inhibitor nelfinavir. These virions also look quite similar to normal HIV particles in size and shape.
Similar articles
- Morphometric analysis of envelope glycoprotein gp120 distribution on HIV-1 virions.
Hart TK, Klinkner AM, Ventre J, Bugelski PJ. Hart TK, et al. J Histochem Cytochem. 1993 Feb;41(2):265-71. doi: 10.1177/41.2.7678271. J Histochem Cytochem. 1993. PMID: 7678271 - Atomic force microscopy investigation of isolated virions of murine leukemia virus.
Kuznetsov YG, Low A, Fan H, McPherson A. Kuznetsov YG, et al. J Virol. 2005 Feb;79(3):1970-4. doi: 10.1128/JVI.79.3.1970-1974.2005. J Virol. 2005. PMID: 15650226 Free PMC article. - Atomic force microscopy imaging of retroviruses: human immunodeficiency virus and murine leukemia virus.
Kuznetsov YG, Victoria JG, Low A, Robinson WE Jr, Fan H, McPherson A. Kuznetsov YG, et al. Scanning. 2004 Sep-Oct;26(5):209-16. doi: 10.1002/sca.4950260409. Scanning. 2004. PMID: 15536976 - Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus.
Chertova E, Bess JW Jr, Crise BJ, Sowder II RC, Schaden TM, Hilburn JM, Hoxie JA, Benveniste RE, Lifson JD, Henderson LE, Arthur LO. Chertova E, et al. J Virol. 2002 Jun;76(11):5315-25. doi: 10.1128/jvi.76.11.5315-5325.2002. J Virol. 2002. PMID: 11991960 Free PMC article. - Atomic force microscopy in imaging of viruses and virus-infected cells.
Kuznetsov YG, McPherson A. Kuznetsov YG, et al. Microbiol Mol Biol Rev. 2011 Jun;75(2):268-85. doi: 10.1128/MMBR.00041-10. Microbiol Mol Biol Rev. 2011. PMID: 21646429 Free PMC article. Review.
Cited by
- Simultaneous Protein and RNA Analysis in Single Extracellular Vesicles, Including Viruses.
Troyer Z, Gololobova O, Koppula A, Liao Z, Horns F, Elowitz MB, Tosar JP, Batish M, Witwer KW. Troyer Z, et al. ACS Nano. 2024 Oct 1;18(39):26568-26584. doi: 10.1021/acsnano.4c03679. Epub 2024 Sep 22. ACS Nano. 2024. PMID: 39306763 Free PMC article. - Administration of anti-HIV-1 broadly neutralizing monoclonal antibodies with increased affinity to Fcγ receptors during acute SHIVAD8-EO infection.
Dias J, Fabozzi G, Fourati S, Chen X, Liu C, Ambrozak DR, Ransier A, Laboune F, Hu J, Shi W, March K, Maximova AA, Schmidt SD, Samsel J, Talana CA, Ernste K, Ko SH, Lucas ME, Radecki PE, Boswell KL, Nishimura Y, Todd JP, Martin MA, Petrovas C, Boritz EA, Doria-Rose NA, Douek DC, Sékaly RP, Lifson JD, Asokan M, Gama L, Mascola JR, Pegu A, Koup RA. Dias J, et al. Nat Commun. 2024 Aug 29;15(1):7461. doi: 10.1038/s41467-024-51848-y. Nat Commun. 2024. PMID: 39198422 Free PMC article. - Recent Advances in Early Diagnosis of Viruses Associated with Gastroenteritis by Biosensors.
Babaei A, Rafiee N, Taheri B, Sohrabi H, Mokhtarzadeh A. Babaei A, et al. Biosensors (Basel). 2022 Jul 8;12(7):499. doi: 10.3390/bios12070499. Biosensors (Basel). 2022. PMID: 35884302 Free PMC article. Review. - Insight into prognostics, diagnostics, and management strategies for SARS CoV-2.
Amara U, Rashid S, Mahmood K, Nawaz MH, Hayat A, Hassan M. Amara U, et al. RSC Adv. 2022 Mar 11;12(13):8059-8094. doi: 10.1039/d1ra07988c. eCollection 2022 Mar 8. RSC Adv. 2022. PMID: 35424750 Free PMC article. Review. - Potent anti-viral activity of a trispecific HIV neutralizing antibody in SHIV-infected monkeys.
Pegu A, Xu L, DeMouth ME, Fabozzi G, March K, Almasri CG, Cully MD, Wang K, Yang ES, Dias J, Fennessey CM, Hataye J, Wei RR, Rao E, Casazza JP, Promsote W, Asokan M, McKee K, Schmidt SD, Chen X, Liu C, Shi W, Geng H, Foulds KE, Kao SF, Noe A, Li H, Shaw GM, Zhou T, Petrovas C, Todd JP, Keele BF, Lifson JD, Doria-Rose NA, Koup RA, Yang ZY, Nabel GJ, Mascola JR. Pegu A, et al. Cell Rep. 2022 Jan 4;38(1):110199. doi: 10.1016/j.celrep.2021.110199. Cell Rep. 2022. PMID: 34986348 Free PMC article.
References
- Bennett, V. 1982. The molecular basis for membrane-cytoskeleton association in human erythrocytes. J. Cell. Biochem. 18:49-65. - PubMed
- Berger, E. A. 1998. And the best picture is—the HIV gp envelope, please! Nat. Struct. Biol. 5:671-674. - PubMed
- Bolognesi, D. P., R. C. Montelaro, H. Frank, and W. Schafer. 1978. Assembly of C-type oncornaviruses. A model. Science 199:183-186. - PubMed
- Braet, F., D. Vermijlen, V. Bossuyt, R. De Zanger, and E. Wisse. 2001. Early detection of cytotoxic events between hepatic natural killer cells and colon carcinoma cells as probed with the atomic force microscope. Ultramicroscopy 89:265-273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous