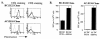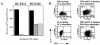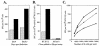Cytolytic CD4(+)-T-cell clones reactive to EBNA1 inhibit Epstein-Barr virus-induced B-cell proliferation - PubMed (original) (raw)
Cytolytic CD4(+)-T-cell clones reactive to EBNA1 inhibit Epstein-Barr virus-induced B-cell proliferation
Sarah Nikiforow et al. J Virol. 2003 Nov.
Abstract
In the absence of immune surveillance, Epstein-Barr virus (EBV)-infected B cells generate neoplasms in vivo and transformed cell lines in vitro. In an in vitro system which modeled the first steps of in vivo immune control over posttransplant lymphoproliferative disease and lymphomas, our investigators previously demonstrated that memory CD4(+) T cells reactive to EBV were necessary and sufficient to prevent proliferation of B cells newly infected by EBV (S. Nikiforow et al., J. Virol. 75:3740-3752, 2001). Here, we show that three CD4(+)-T-cell clones reactive to the latent EBV antigen EBNA1 also prevent the proliferation of newly infected B cells from major histocompatibility complex (MHC) class II-matched donors, a crucial first step in the transformation process. EBNA1-reactive T-cell clones recognized B cells as early as 4 days after EBV infection through an HLA-DR-restricted interaction. They secreted Th1-type and Th2-type cytokines and lysed EBV-transformed established lymphoblastoid cell lines via a Fas/Fas ligand-dependent mechanism. Once specifically activated, they also caused bystander regression and bystander killing of non-MHC-matched EBV-infected B cells. Since EBNA1 is recognized by CD4(+) T cells from nearly all EBV-seropositive individuals and evades detection by CD8(+) T cells, EBNA1-reactive CD4(+) T cells may control de novo expansion of B cells following EBV infection in vivo. Thus, EBNA1-reactive CD4(+)-T-cell clones may find use as adoptive immunotherapy against EBV-related lymphoproliferative disease and many other EBV-associated tumors.
Figures
FIG. 1.
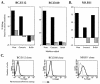
CD4+-T-cell clones recognize EBNA1 presented on MHC II-matched DCs. (A) FACS analysis of T-cell clones incubated with fluorochrome-conjugated antibodies against CD4 (left panels; thick lines), CD8 (right panels; thick lines), or isotype control antibodies (thin, dotted lines). (B) IFN-γ secretion by a representative BC clone, BC.E122, and a representative AC clone, AC.E116, in response to DCs loaded with _E. coli_-derived rEBNA1 (aa 458 to 641) or PCNA proteins. HLA-DR7-matched (BC.E122 panel) or autologous (AC.E116 panel) DCs were loaded with protein at the time of maturation and seeded in ELISpot wells. Results represent the average of triplicate samples; standard errors of the means are shown.
FIG. 2.
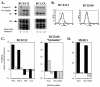
CD4+-T-cell clones raised against EBNA1 express both Th1- and Th2-type cytokines. (A) Secretion of IFN-γ (solid bars), IL-4 (stippled bars), and IL-5 (hatched bars) by 104 BC.E112 (left panel) or BC.E160 (right panel) cells in response to 104 autologous LCLs in ELISpot assays. SFC counts represent the average of duplicate samples. (B) Intracellular staining for production of IFN-γ (FL1 fluorescence) or IL-4 (FL3 fluorescence) by the BC.E160 cells. The BC.E160 clone was stimulated for 18 h with PMA and ionomycin or autologous LCLs. Plots on the left represent cells stained with antibody to IFN-γ alone (upper left plot, lower right quadrant) or antibody to IL-4 alone (lower left plot, upper left quadrant). Plots on the right represent cells stained with antibodies to both IFN-γ and IL-4 (upper right quadrants).
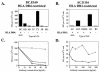
FIG. 3.
EBNA1-reactive CD4+-T-cell clones recognize MHC II-matched LCLs. (A and B) Recognition of LCLs bearing different MHC II molecules by EBNA1-reactive CD4+-T-cell clones in IFN-γ ELISpot assays; 104 BC.E160 cells were added to 104 LCLs from donors matched or mismatched for the HLA-DR4 molecule (A), or 105 AC.E116 cells were added to 105 LCLs or an HLA-DR1-positive Hodgkin's lymphoma (RPMI6666) (B). SFC counts represent the average of triplicate samples; standard errors of the means are displayed. (C and D) Effect of antibodies against MHC I and MHC II on IFN-γ secretion by clones incubated with MHC II-matched LCLs in ELISpot assays. Antibodies to MHC I molecules (dotted lines), to MHC II molecules (solid line, open symbols), or a mixture of antibodies to MHC I and II (solid lines, filled symbols) were added at the indicated concentrations to LCLs and clones. LCL targets were HLA-DR4+/DR7− (x's) added at 5 × 103 per well to BC.E160 cells (C) or HLA-DR1+ (triangles) added at 5 × 104 per well to AC.E116 cells (D). SFCs represent the average of duplicate samples; data are representative of at least two experiments performed with each set of clones.
FIG. 4.
EBNA1-reactive CD4+-T-cell clones secrete IFN-γ in response to B cells newly infected with EBV. (A) IFN-γ secretion in an ELISpot assay by a 2:1 mixture of clone BC.E112 cells and freshly EBV-infected (solid bars) or mock-inoculated (stippled bars) B cells after coculture for 4 and 6.5 days. (B) IFN-γ secretion by clone BC.E112 and clone JB.flu10 cells exposed to B cells for the 18-h duration of an ELISpot assay. The B cells had been cultured with EBV (solid bars) or a mock inoculum (stippled bars) for 4 days prior to exposure to the clones. Results represent the average of duplicate samples and are representative of at least two experiments performed on B cells from different donors. (C) IFN-γ secretion by clone BC.E122 in response to purified B cells cultured with EBV for 4 days. B cells were incubated with antibodies against MHC I or II at 100 μg/ml before addition of the T-cell clones. No antibody addition (solid line, filled symbols), addition of antibody to MHC I (dashed line, open symbols), and addition of antibody to MHC II (solid line, open symbols) are represented.
FIG. 5.
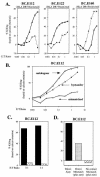
EBNA1-reactive CD4+-T-cell clones from donor BC reduce outgrowth of CD23+ B cells in EBV-infected cultures derived from the autologous donor. Shown are the percentage (A and C) and number (B) of CD23+ B cells remaining after 16 to 18 days in culture. (A and B) Serial 10-fold dilutions of two clones, the HLA-DR4-restricted BC.E160 (gray bars) and the HLA-DR7-restricted BC.E122 clone (stippled bars), were added to 106 EBV-infected CD3-depleted PBMCs/ml from autologous donor BC cells. Hatched bars represent the outgrowth of CD3-depleted target cells without the addition of T-cell clones at the time of infection on day 0. White bars represent outgrowth in cultures of EBV-infected, autologous, mixed PBMCs. (C) AC clones fail to inhibit CD23+ B-cell outgrowth. Equal numbers of the AC.E116 and AC.E1111 clones, the JB.flu10 clone, or mixed autologous CD4+ T cells were added to 1.5 × 106 EBV-infected CD3-depleted PBMCs/ml from donor AC cells on day 0. The percent change which addition of T cells effected on CD23+ B-cell outgrowth is listed to the right of the graphs as the percent regression.
FIG. 6.
EBNA1-reactive CD4+-T-cell clones inhibit outgrowth of CD23+ B cells in EBV-infected cultures derived from MHC II-matched donors and from mismatched bystander B cells. Shown is the percentage of CD23+ B cells remaining after 18 days in culture. (A and B) CD4+-T-cell clone BC.E112 inhibited outgrowth of EBV-infected B cells from HLA-DR7+ but not HLA-DR7− donors. Equal numbers of autologous mixed CD4+ cells (solid bars), clone BC.E112 cells (stippled bars), or no T cells (hatched bar) were added to 106 EBV-infected B-cell targets/ml derived from HLA-DR7+ or HLA-DR7− donors on day 0. (B) Tenfold dilutions of autologous mixed CD4+ T cells (solid bars) or the BC.E112 clone (stippled bars) were added to 106 EBV-infected B-cell targets/ml (hatched bars) from an HLA-DR7+ matched donor on day 0. (C) Clone BC.E160 inhibited outgrowth of EBV-infected B cells from an HLA-DR4+ donor. Equal numbers of the HLA-DR7-restricted BC.E112 and BC.E122 clones, the HLA-DR4-restricted BC.E160 clone, or the JB.flu10 clone were added to 1.5 × 106 EBV-infected HLA-DR4+ B-cell targets/ml on day 0. Cultures of mixed PBMCs derived from the HLA-DR4+ B-cell donor and seeded at 1.5 × 106/ml were cultured as well (solid bar). (D) Bystander inhibition of outgrowth of HLA-DR7-negative mismatched B cells. Equal numbers of the BC.E112 clone or the BC.E122 clone were added to 106 HLA-DR7-negative mismatched EBV-infected B cell targets/ml (solid bars) on day 0. Parallel cultures contained both mismatched and 106 HLA-DR7-positive matched B-cell targets/ml (hatched bars) in the presence of clone BC.E122, clone BC.E112, or no T cells. Mismatched HLA-DR7-negative targets were selectively analyzed. Comparison of the hatched bars to the solid bars demonstrates the extent of inhibition exerted over mismatched CD23+ B-cell targets due to the presence of matched HLA-DR7-positive targets. The percent change which addition of MHC-matched B cells effected on CD23+ B-cell outgrowth is listed to the right of the graphs as percent bystander effect.
FIG. 7.
EBNA1-reactive CD4+-T-cell clones are cytotoxic following direct contact with MHC II-matched LCLs and with mismatched bystander LCLs. (A) Retention of calcein dye in MHC II-matched and -mismatched LCLs exposed for 19 h to each of three BC clones. LCL targets were HLA-DR4−/DR7+ (solid line, squares) or HLA-DR4+/DR7− (dashed line, x's). (B) Retention of calcein dye 18 h after addition of the BC.E112 clone to autologous LCLs (solid line, squares), MHC-mismatched HLA-DR7− LCLs (solid lines, x's), or a combination of autologous and mismatched LCLs (dotted line, filled squares). In cultures where LCLs were combined, only the HLA-DR7− mismatched LCLs were labeled with calcein and analyzed. (C) Cytotoxicity measured by retention of calcein dye by autologous LCLs after 18 h of direct contact with the BC.E112 clone (solid bars) or by autologous LCLs separated from the same BC.E112-LCL cocultures by a porous membrane (hatched bars). (D) Cytotoxicity against autologous LCLs (solid bars) or against bystander MHC II-mismatched LCLs (stippled bars) after 18 h of direct contact with clone BC.E112 or against the same mismatched LCLs separated from BC.E112-autologous LCL cultures by a porous membrane (hatched bar).
FIG. 8.
EBNA1-reactive CD4+-T-cell clones do not utilize perforin to induce cytolysis of LCLs. (A) Cytotoxicity against autologous (solid bars) or MHC II-mismatched (stippled bars) LCL targets after 18 h in culture with BC.E112 or BC.E160 cells at a 1:1 ratio. Prior to exposure to LCLs, clones were incubated with concanamycin A (ConcanA), brefeldin A (BrfdA), or culture medium alone (none). (B) Loss of calcein dye by MHC I-matched LCL targets with (solid bars) or without (stippled bars) addition of EBNA3A antigenic peptide after 3 h in culture with MS.B11 CD8+-T-cell clones at a 1:1 ratio. (C) Intracellular staining with antibody to perforin. BC EBNA1-reactive CD4+ clones were stimulated with autologous LCLs; the EBNA3A-specific CD8+-T-cell clone MS.B11 was stimulated with peptide-loaded, MHC I-matched LCL (solid lines). Staining with a PE-conjugated isotype control is shown by the dotted lines.
FIG. 9.
EBNA1-reactive CD4+-T-cell clones express Fas ligand (FasL) when activated and induce cytolysis of LCLs via a Fas and FasL interaction. (A) RPA performed at the indicated times after stimulation of the BC.E112 clone or autologous LCLs with PMA and ionomycin. Relevant transcripts are indicated by arrows. L32 represents a housekeeping gene. (B) Intracellular staining with NOK-2 antibody to Fas ligand expressed by the BC clones after stimulation with autologous LCL (solid lines). Staining with an isotype control antibody and the secondary FITC-conjugated anti-murine IgG antibody is shown by the dotted lines. (C) Loss of intracellular calcein dye by autologous LCLs (solid bars) or MHC II-mismatched (stippled bars) LCL targets alone after 18 h in culture with BC.E112 clones (E:T ratio of 2:1) or BC.E160 clones (E:T ratio of 1:1). The panel entitled “BC.E160 bystander” compares killing of autologous LCLs (solid bars), MHC II-mismatched LCLs (stippled bars), and MHC II-mismatched LCLs in the presence of autologous LCLs (hatched bars). (D) Loss of calcein dye by MHC I-matched, antigen-loaded (solid bars) or nonloaded (stippled bars) LCLs after 3 h in culture with CD8+-T-cell clone MS.B11 at an E:T ratio of 1:1. In panels C and D, T cells and LCLs were incubated with culture medium (none), the NOK-2 antibody to Fas Ligand, the ZB4 neutralizing antibody to Fas, a combination of both antibodies (NOK-2/ZB4), or the pan-caspase inhibitor zVAD-fmk.
Similar articles
- Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1.
Münz C, Bickham KL, Subklewe M, Tsang ML, Chahroudi A, Kurilla MG, Zhang D, O'Donnell M, Steinman RM. Münz C, et al. J Exp Med. 2000 May 15;191(10):1649-60. doi: 10.1084/jem.191.10.1649. J Exp Med. 2000. PMID: 10811859 Free PMC article. - CD4+ T cells inhibit growth of Epstein-Barr virus-transformed B cells through CD95-CD95 ligand-mediated apoptosis.
Wilson AD, Redchenko I, Williams NA, Morgan AJ. Wilson AD, et al. Int Immunol. 1998 Aug;10(8):1149-57. doi: 10.1093/intimm/10.8.1149. Int Immunol. 1998. PMID: 9723701 - T cells specific for different latent and lytic viral proteins efficiently control Epstein-Barr virus-transformed B cells.
Nowakowska J, Stuehler C, Egli A, Battegay M, Rauser G, Bantug GR, Brander C, Hess C, Khanna N. Nowakowska J, et al. Cytotherapy. 2015 Sep;17(9):1280-91. doi: 10.1016/j.jcyt.2015.06.003. Cytotherapy. 2015. PMID: 26276009 - Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products.
Ressing ME, Horst D, Griffin BD, Tellam J, Zuo J, Khanna R, Rowe M, Wiertz EJ. Ressing ME, et al. Semin Cancer Biol. 2008 Dec;18(6):397-408. doi: 10.1016/j.semcancer.2008.10.008. Epub 2008 Oct 25. Semin Cancer Biol. 2008. PMID: 18977445 Review. - Contrasting roles of dendritic cells and B cells in the immune control of Epstein-Barr virus.
Bickham K, Münz C. Bickham K, et al. Curr Top Microbiol Immunol. 2003;276:55-76. doi: 10.1007/978-3-662-06508-2_3. Curr Top Microbiol Immunol. 2003. PMID: 12797443 Review.
Cited by
- Cytolytic CD4 cells: Direct mediators in infectious disease and malignancy.
Brown DM. Brown DM. Cell Immunol. 2010;262(2):89-95. doi: 10.1016/j.cellimm.2010.02.008. Epub 2010 Feb 24. Cell Immunol. 2010. PMID: 20236628 Free PMC article. Review. - A CD4+ T Cell-NK Cell Axis of Gammaherpesvirus Control.
Lawler C, Stevenson PG. Lawler C, et al. J Virol. 2020 Jan 17;94(3):e01545-19. doi: 10.1128/JVI.01545-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694958 Free PMC article. - Cytotoxic CD4 T Cells-Friend or Foe during Viral Infection?
Juno JA, van Bockel D, Kent SJ, Kelleher AD, Zaunders JJ, Munier CM. Juno JA, et al. Front Immunol. 2017 Jan 23;8:19. doi: 10.3389/fimmu.2017.00019. eCollection 2017. Front Immunol. 2017. PMID: 28167943 Free PMC article. Review. - Fighting Viral Infections and Virus-Driven Tumors with Cytotoxic CD4+ T Cells.
Muraro E, Merlo A, Martorelli D, Cangemi M, Dalla Santa S, Dolcetti R, Rosato A. Muraro E, et al. Front Immunol. 2017 Feb 27;8:197. doi: 10.3389/fimmu.2017.00197. eCollection 2017. Front Immunol. 2017. PMID: 28289418 Free PMC article. Review. - Macroautophagy Proteins Assist Epstein Barr Virus Production and Get Incorporated Into the Virus Particles.
Nowag H, Guhl B, Thriene K, Romao S, Ziegler U, Dengjel J, Münz C. Nowag H, et al. EBioMedicine. 2014 Nov 8;1(2-3):116-25. doi: 10.1016/j.ebiom.2014.11.007. eCollection 2014 Dec. EBioMedicine. 2014. PMID: 26137519 Free PMC article.
References
- Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. - PubMed
- Ando, K., K. Hiroishi, T. Kaneko, T. Moriyama, Y. Muto, N. Kayagaki, H. Yagita, K. Okumura, and M. Imawari. 1997. Perforin, Fas/Fas ligand, and TNF-alpha pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J. Immunol. 158:5283-5291. - PubMed
- Azim, T., and D. H. Crawford. 1988. Lymphocytes activated by the Epstein-Barr virus to produce immunoglobulin do not express CD23 or become immortalized. Int. J. Cancer 42:23-28. - PubMed
- Babcock, G. J., D. Hochberg, and A. D. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497-506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM07205/GM/NIGMS NIH HHS/United States
- CA16038/CA/NCI NIH HHS/United States
- R01 CA012055/CA/NCI NIH HHS/United States
- T32 GM007205/GM/NIGMS NIH HHS/United States
- P01 CA016038/CA/NCI NIH HHS/United States
- CA12055/CA/NCI NIH HHS/United States
- R37 CA012055/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous