Inflammation is detrimental for neurogenesis in adult brain - PubMed (original) (raw)
Inflammation is detrimental for neurogenesis in adult brain
Christine T Ekdahl et al. Proc Natl Acad Sci U S A. 2003.
Abstract
New hippocampal neurons are continuously generated in the adult brain. Here, we demonstrate that lipopolysaccharide-induced inflammation, which gives rise to microglia activation in the area where the new neurons are born, strongly impairs basal hippocampal neurogenesis in rats. The increased neurogenesis triggered by a brain insult is also attenuated if it is associated with microglia activation caused by tissue damage or lipopolysaccharide infusion. The impaired neurogenesis in inflammation is restored by systemic administration of minocycline, which inhibits microglia activation. Our data raise the possibility that suppression of hippocampal neurogenesis by activated microglia contributes to cognitive dysfunction in aging, dementia, epilepsy, and other conditions leading to brain inflammation.
Figures
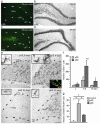
Fig. 1.
Inflammation impairs basal hippocampal neurogenesis. (_a_-c) Immunohistochemical staining for ED1-positive activated microglia in the DG after 28 days of intracortical vehicle (a) or LPS (b) infusions, and number of activated microglia in the SGZ/GCL in the same treatment groups (c). (d) A newly formed BrdUrd/NeuN double-labeled neuron in the SGZ is visualized by using confocal microscopy in an orthogonal projection composed of 19 optical _z_-planes (0.5 μm thick). (e) The number of BrdUrd/NeuN double-labeled new neurons in the SGZ/GCL after 28 days of LPS or vehicle infusion (14 days after the last BrdUrd injection). (f) Correlation between number of ED1-positive and BrdUrd/NeuN double-labeled cells. Data are the number of cells per section. n = 6 and 10 for vehicle- and LPS-treated rats, respectively. Shown are means ± SEM. *, P < 0.05. Hilus, dentate hilus. Dotted lines depict SGZ. (Scale bar = 20 μm in b and 7.2 μm in d.)
Fig. 2.
Hippocampal neurogenesis is impaired after brain insult associated with tissue damage and inflammation. (_a_-d) Neurodegeneration as visualized with Fluoro-Jade staining (a and c) and NeuN immunoreactivity (b and d) in the DG 35 days after SE. Note more damage and fewer remaining neurons in the dentate hilus after generalized SE (gSE) as compared with partial SE (pSE). (e) The distribution of ED1-positive, activated microglia in the DG 6 and 35 days after partial or generalized SE. (Left Inset) Shown are higher magnification of activated microglia. (Right Inset) Shown are ED1-positive (green) cells surrounding BrdUrd-positive (red) cells in the SGZ. (f) The number of ED1-positive microglia in the SGZ/GCL 2, 6-8, and 35 days after SE. (g) The number of BrdUrd/NeuN double-labeled new neurons in the SGZ/GCL 35 days after SE (28 days after BrdUrd injections). Data are number of cells per section. Shown are means ± SEM. *, P < 0.05 (compared with pSE in f). #, P < 0.05 compared with 35 days. In f, n = 6(3 + 3), 21 (14 + 7), and 13 (8 + 5) for the 2-day, 6- to 8-day, and 35-day groups, respectively, with individual numbers for pSE and gSE in parentheses. In g, n = 5, 8, and 5 for nonstimulated controls, pSE, and gSE, respectively. Hilus, dentate hilus. Dotted lines depict SGZ. (Scale bar = 100 μm in _a_-e and 10 μm in Right Inset in e.)
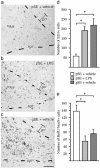
Fig. 3.
Inflammation causes impairment of hippocampal neurogenesis after brain insult not associated with tissue damage. (_a_-c) Immunohistochemical staining of ED1-positive activated microglia in the SGZ/GCL after intracortical vehicle or LPS infusions during 28 days after partial SE (pSE) or generalized SE (gSE). (d) The number of ED1-positive cells in the DG after SE. Note that the distribution and number of ED1-positive microglia in the SGZ is similar in partial SE plus LPS and generalized SE plus vehicle animals whereas there are very few cells in the partial SE plus vehicle rats. (e) The number of BrdUrd/NeuN-positive new neurons in the SGZ/GCL 28 days after LPS or vehicle treatment after SE (21 days after BrdUrd injections). Data are number of cells per section. Shown are means ± SEM. *, P < 0.05. n = 16, 15, and 5 for pSE plus LPS, pSE plus vehicle, and gSE plus vehicle, respectively. Hilus, dentate hilus. Dotted lines depict SGZ. (Scale bar = 100 μm.)
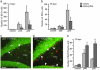
Fig. 4.
Minocycline prevents inflammation-mediated suppression of hippocampal neurogenesis. (a and b) The number of ED1-positive, activated microglia in the SGZ/GCL in nonstimulated controls, and 6 days (a) and 35 days (b) after generalized SE (gSE) and partial SE (pSE) with or without minocycline treatment. (c and d) The distribution of BrdUrd (red) and NeuN (green) cells in the DG at 35 days after gSE with and without minocycline treatment. Arrowheads depict BrdUrd/NeuN-double-labeled new neurons. (e) The number of new neurons in the SGZ/GCL in nonstimulated controls and 35 days after gSE and pSE (28 days after BrdUrd injections). In a, n = 19 (5 + 9 + 5) and 14 (4 + 7 + 3); in b, n = 13 (4 + 5 + 4) and 14 (5 + 4 + 5); and in e, n = 13 (4 + 5 + 4) and 14 (5 + 4 + 5) for minocycline and vehicle, respectively, with individual numbers for nonstimulated controls, pSE, and gSE in brackets. Data are number of cells per section. Shown are means ± SEM. *, P < 0.05 compared with vehicle-treated animals. Hilus, dentate hilus. Scale bar = 50 μm.
Similar articles
- Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia.
Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Hua XY, et al. Eur J Neurosci. 2005 Nov;22(10):2431-40. doi: 10.1111/j.1460-9568.2005.04451.x. Eur J Neurosci. 2005. PMID: 16307586 - Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFβ1 downregulation.
Graciarena M, Depino AM, Pitossi FJ. Graciarena M, et al. Brain Behav Immun. 2010 Nov;24(8):1301-9. doi: 10.1016/j.bbi.2010.06.005. Epub 2010 Jun 20. Brain Behav Immun. 2010. PMID: 20600816 - Roles of astrocytes and microglia in seizure-induced aberrant neurogenesis in the hippocampus of adult rats.
Yang F, Liu ZR, Chen J, Zhang SJ, Quan QY, Huang YG, Jiang W. Yang F, et al. J Neurosci Res. 2010 Feb 15;88(3):519-29. doi: 10.1002/jnr.22224. J Neurosci Res. 2010. PMID: 19774666 - Research update: neurogenesis in adult brain and neuropsychiatric disorders.
Elder GA, De Gasperi R, Gama Sosa MA. Elder GA, et al. Mt Sinai J Med. 2006 Nov;73(7):931-40. Mt Sinai J Med. 2006. PMID: 17195878 Review. - Brain inflammation and adult neurogenesis: the dual role of microglia.
Ekdahl CT, Kokaia Z, Lindvall O. Ekdahl CT, et al. Neuroscience. 2009 Feb 6;158(3):1021-9. doi: 10.1016/j.neuroscience.2008.06.052. Epub 2008 Jul 3. Neuroscience. 2009. PMID: 18662748 Review.
Cited by
- Selective Ablation of BDNF from Microglia Reveals Novel Roles in Self-Renewal and Hippocampal Neurogenesis.
Harley SBR, Willis EF, Shaikh SN, Blackmore DG, Sah P, Ruitenberg MJ, Bartlett PF, Vukovic J. Harley SBR, et al. J Neurosci. 2021 May 12;41(19):4172-4186. doi: 10.1523/JNEUROSCI.2539-20.2021. Epub 2021 Mar 30. J Neurosci. 2021. PMID: 33785644 Free PMC article. - Ethyl pyruvate protects against blood-brain barrier damage and improves long-term neurological outcomes in a rat model of traumatic brain injury.
Shi H, Wang HL, Pu HJ, Shi YJ, Zhang J, Zhang WT, Wang GH, Hu XM, Leak RK, Chen J, Gao YQ. Shi H, et al. CNS Neurosci Ther. 2015 Apr;21(4):374-84. doi: 10.1111/cns.12366. Epub 2014 Dec 23. CNS Neurosci Ther. 2015. PMID: 25533312 Free PMC article. - Harnessing the Stem Cell Niche in Regenerative Medicine: Innovative Avenue to Combat Neurodegenerative Diseases.
Velikic G, Maric DM, Maric DL, Supic G, Puletic M, Dulic O, Vojvodic D. Velikic G, et al. Int J Mol Sci. 2024 Jan 12;25(2):993. doi: 10.3390/ijms25020993. Int J Mol Sci. 2024. PMID: 38256066 Free PMC article. Review. - NLRP3 Inflammasome Inhibition After Pilocarpine-Induced Status Epilepticus Attenuates Chronic Inflammation in Epileptic Mice.
Wang L, Wang K, Chen Y, Zhang X, Xu W, Dong Z, Wang Y. Wang L, et al. J Inflamm Res. 2024 Sep 7;17:6143-6158. doi: 10.2147/JIR.S469451. eCollection 2024. J Inflamm Res. 2024. PMID: 39262652 Free PMC article. - Galectin-9 Promotes Neuronal Restoration via Binding TLR-4 in a Rat Intracerebral Hemorrhage Model.
Liang T, Ma C, Wang T, Deng R, Ding J, Wang W, Xu Z, Li X, Li H, Sun Q, Shen H, Wang Z, Chen G. Liang T, et al. Neuromolecular Med. 2021 Jun;23(2):267-284. doi: 10.1007/s12017-020-08611-5. Epub 2020 Aug 31. Neuromolecular Med. 2021. PMID: 32865657
References
- Cameron, H. A. & McKay, R. D. (2001) J. Comp. Neurol. 435, 406-417. - PubMed
- Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T. & Gould, E. (2001) Nature 410, 372-376. - PubMed
- Santarelli, L., Saxe, M., Gross, C., Surget, A., Battaglia, F., Dulawa, S., Weisstaub, N., Lee, J., Duman, R., Arancio, O., Belzung, C. & Hen, R. (2003) Science 301, 805-809. - PubMed
- Zitnik, G. & Martin, G. M. (2002) J. Neurosci. Res. 70, 258-263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources