A comparative study of motor-protein motions by using a simple elastic-network model - PubMed (original) (raw)
Comparative Study
. 2003 Nov 11;100(23):13253-8.
doi: 10.1073/pnas.2235686100. Epub 2003 Oct 29.
Affiliations
- PMID: 14585932
- PMCID: PMC263771
- DOI: 10.1073/pnas.2235686100
Comparative Study
A comparative study of motor-protein motions by using a simple elastic-network model
Wenjun Zheng et al. Proc Natl Acad Sci U S A. 2003.
Abstract
In this work, we report on a study of the structure-function relationships for three families of motor proteins, including kinesins, myosins, and F1-ATPases, by using a version of the simple elastic-network model of large-scale protein motions originally proposed by Tirion [Tirion, M. (1996) Phys. Rev. Lett. 77, 1905-1908]. We find a surprising dichotomy between kinesins and the other motor proteins (myosins and F1-ATPase). For the latter, there exist one or two dominant lowest-frequency modes (one for myosin, two for F1-ATPase) obtained from normal-mode analysis of the elastic-network model, which overlap remarkably well with the measured conformational changes derived from pairs of solved crystal structures in different states. Furthermore, we find that the computed global conformational changes induced by the measured deformation of the nucleotide-binding pocket also overlap well with the measured conformational changes, which is consistent with the "nucleotide-binding-induced power-stroke" scenario. In contrast, for kinesins, this simplicity breaks down. Multiple modes are needed to generate the measured conformational changes, and the computed displacements induced by deforming the nucleotide-binding pocket also overlap poorly with the measured conformational changes, and are insufficient to explain the large-scale motion of the relay helix and the linker region. This finding may suggest the presence of two different mechanisms for myosins and kinesins, despite their strong evolutionary ties and structural similarities.
Figures
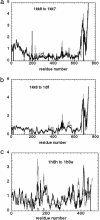
Fig. 1.
The overlap of the measured conformational change with the lowest 50 normal modes for myosins and ATPases: myosin 1KK8 (with truncated lever) to 1KK7 (a); 1KK8 (with truncated lever) to 1DFL (b); and F1-ATPase PDB ID code 1H8H (chain E) to PDB ID code1H8E (chain E) (c). The thick curve is the overlap per mode, and the thin curve is the cumulative overlap using all modes below a given mode. It is observed that there exists a single mode that dominates the measured conformational change in all three cases.
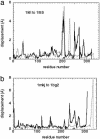
Fig. 2.
The amplitude of the computed displacement induced by the pocket deformation versus the measured conformational change for myosin 1KK8 (with truncated lever) to 1KK7 (a) myosin 1KK8 (with truncated lever); to 1DFL (b); and F1-ATPase 1H8H chain E to 1H8E chain E (c). Thick curve, amplitude of the measured conformational change; thin dotted curve, amplitude of the displacement induced by the nucleotide binding pocket deformation; boxes, nucleotide-binding sites.
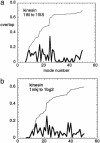
Fig. 3.
The overlap of the measured conformational change with the lowest 50 normal modes for kinesins:1I6I to 1I5S (a) and 1MKJ to 1BG2 (b). Thick curve, overlap per mode; thin curve, cumulative overlap using all modes below a given mode. It is observed that there does not exist a single mode that dominates the measured conformational change.
Fig. 4.
The amplitude of the computed displacement induced by the pocket deformation versus the measured conformational change for kinesins: 1I6I to 1I5S (a) and 1MKJ to 1BG2 (b). Thick lines, amplitude of the measured conformational change; thin dotted lines, amplitude of the displacement induced by the nucleotide-binding pocket deformation; boxes, nucleotide-binding sites.
Similar articles
- Structural basis for power stroke vs. Brownian ratchet mechanisms of motor proteins.
Hwang W, Karplus M. Hwang W, et al. Proc Natl Acad Sci U S A. 2019 Oct 1;116(40):19777-19785. doi: 10.1073/pnas.1818589116. Epub 2019 Sep 10. Proc Natl Acad Sci U S A. 2019. PMID: 31506355 Free PMC article. - Probing the local dynamics of nucleotide-binding pocket coupled to the global dynamics: myosin versus kinesin.
Zheng W, Brooks BR. Zheng W, et al. Biophys J. 2005 Jul;89(1):167-78. doi: 10.1529/biophysj.105.063305. Epub 2005 May 6. Biophys J. 2005. PMID: 15879477 Free PMC article. - Cooperativity in the motor activities of the ATP-fueled molecular motors.
Liu MS, Todd BD, Sadus RJ. Liu MS, et al. Biochim Biophys Acta. 2005 Sep 25;1752(2):111-23. doi: 10.1016/j.bbapap.2005.06.013. Biochim Biophys Acta. 2005. PMID: 16140597 - Kinesin: switch I & II and the motor mechanism.
Kull FJ, Endow SA. Kull FJ, et al. J Cell Sci. 2002 Jan 1;115(Pt 1):15-23. doi: 10.1242/jcs.115.1.15. J Cell Sci. 2002. PMID: 11801720 Review. - Toward intelligent molecular machines: directed motions of biological and artificial molecules and assemblies.
Kinbara K, Aida T. Kinbara K, et al. Chem Rev. 2005 Apr;105(4):1377-400. doi: 10.1021/cr030071r. Chem Rev. 2005. PMID: 15826015 Review. No abstract available.
Cited by
- Impact of phylogeny on the inference of functional sectors from protein sequence data.
Dietler N, Abbara A, Choudhury S, Bitbol AF. Dietler N, et al. PLoS Comput Biol. 2024 Sep 23;20(9):e1012091. doi: 10.1371/journal.pcbi.1012091. eCollection 2024 Sep. PLoS Comput Biol. 2024. PMID: 39312591 Free PMC article. - Atomistic simulation approach to a continuum description of self-assembled beta-sheet filaments.
Park J, Kahng B, Kamm RD, Hwang W. Park J, et al. Biophys J. 2006 Apr 1;90(7):2510-24. doi: 10.1529/biophysj.105.074906. Epub 2006 Jan 13. Biophys J. 2006. PMID: 16415051 Free PMC article. - Making ATP.
Xing J, Liao JC, Oster G. Xing J, et al. Proc Natl Acad Sci U S A. 2005 Nov 15;102(46):16539-46. doi: 10.1073/pnas.0507207102. Epub 2005 Oct 10. Proc Natl Acad Sci U S A. 2005. PMID: 16217018 Free PMC article. - Structural basis for power stroke vs. Brownian ratchet mechanisms of motor proteins.
Hwang W, Karplus M. Hwang W, et al. Proc Natl Acad Sci U S A. 2019 Oct 1;116(40):19777-19785. doi: 10.1073/pnas.1818589116. Epub 2019 Sep 10. Proc Natl Acad Sci U S A. 2019. PMID: 31506355 Free PMC article. - Collective variable approaches for single molecule flexible fitting and enhanced sampling.
Vashisth H, Skiniotis G, Brooks CL 3rd. Vashisth H, et al. Chem Rev. 2014 Mar 26;114(6):3353-65. doi: 10.1021/cr4005988. Epub 2014 Jan 21. Chem Rev. 2014. PMID: 24446720 Free PMC article. Review. No abstract available.
References
- Vale, R. & Milligan, R. (2000) Science 288, 88-95. - PubMed
- Xing, J., Wriggers, W., Jefferson, G. M., Stein, R., Cheung, H. C. & Rosenfeld, S. S. (2000) J. Biol. Chem. 275, 35413-35423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources