Deletion of ghrelin impairs neither growth nor appetite - PubMed (original) (raw)
Deletion of ghrelin impairs neither growth nor appetite
Yuxiang Sun et al. Mol Cell Biol. 2003 Nov.
Abstract
Pharmacological studies show that ghrelin stimulates growth hormone release, appetite, and fat deposition, but ghrelin's physiological role in energy homeostasis has not been established. Ghrelin was also proposed to regulate leptin and insulin release and to be important for the normal function of stomach, heart, kidney, lung, testis, and placenta. To help determine a definable physiological role for ghrelin, we generated ghrelin-null mice. In contrast to predictions made from the pharmacology of ghrelin, ghrelin-null mice are not anorexic dwarfs; their size, growth rate, food intake, body composition, reproduction, gross behavior, and tissue pathology are indistinguishable from wild-type littermates. Fasting produces identical decreases in serum leptin and insulin in null and wild-type mice. Ghrelin-null mice display normal responses to starvation and diet-induced obesity. As in wild-type mice, the administration of exogenous ghrelin stimulates appetite in null mice. Our data show that ghrelin is not critically required for viability, fertility, growth, appetite, bone density, and fat deposition and not likely to be a direct regulator of leptin and insulin. Therefore, antagonists of ghrelin are unlikely to have broad utility as antiobesity agents.
Figures
FIG. 1.
(A) Schematic diagram of the derivation of _ghrelin_−/− mice by homologous recombination. The wild-type allele is shown at the top, and five exons are indicated by a vertical line or rectangular boxes. The targeting construct is shown below. (B) Southern blot analysis of DNA from mice derived from mating heterozygotes.
FIG. 2.
(A) RT-PCR analysis of ghrelin and lacZ mRNA expression. Lanes: S, stomach; B, brain. (B) Immunofluorescence analysis of ghrelin and expression of β-galactosidase activity in the stomach of 8-week-old mice. Magnification, ×8.
FIG. 3.
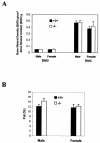
Bone density (A) and body composition (percent fat) (B) in 8-week-old mice (n = 6, P > 0.05 [ghrelin+/+ versus _ghrelin_−/− mice]).
FIG. 4.
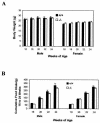
_ghrelin_−/− mice exhibit normal growth rate and normal food intake. (A) Body weight; (B) cumulative food intake. Mice were 16 weeks old at the beginning of the study, and data were collected every other week (n = 8, P > 0.05 [ghrelin+/+ versus _ghrelin_−/− mice]).
FIG. 5.
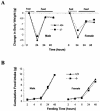
Changes in body weight (A) and food intake (B) during fasting and refeeding. Twenty-week-old mice were fasted for 24 h and then allowed to eat. Body weight was measured before and after fasting and at 24 and 48 h after food was given. Cumulative food intake was measured 2, 4, 6, 24, and 48 h after food was given. The results were plotted according to the mean of each datum point (n = 5, P > 0.05 (ghrelin+/+ versus _ghrelin_−/− mice]).
FIG. 6.
ghrelin−/− mice exhibit a normal orexigenic response to exogenous ghrelin. Cumulative food intake was measured in 12-week-old mice every 30 min for the duration of the experiment. Saline alone (100 μl) or saline containing 10 μg of ghrelin (Phoenix Pharmaceuticals, Inc.) was injected intraperitoneally into each mouse. Mice were first treated with saline and then 0.5 and 1.5 h later with saline containing ghrelin. For each gender group (n = 5), P < 0.05 (✽) in males and P < 0.001 in females (✽✽) (saline versus ghrelin).
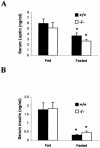
FIG. 7.
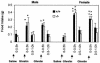
Serum leptin and insulin concentrations in 18-week-old fed or fasted male mice (n = 5). Blood was collected between 8 and 9 a.m. Mice were fasted for 48 h, which produced a fall in leptin and insulin in each genotype (✽, P < 0.05 [fed versus fasted] for both _ghrelin_+/+ and _ghrelin_−/− mice). There was no significant difference in the levels of leptin and insulin between each genotype whether they were fed or fasted (_P_ > 0.05 [ghrelin+/+ versus _ghrelin_−/− mice]).
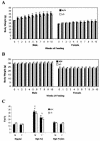
FIG. 8.
ghrelin−/− mice exhibit normal susceptibility to diet-induced obesity. Twenty-week-old mice were fed either a high-fat (A) or a high-protein (B) diet for 10 weeks. M, male (n = 7); F, female (n = 7). Body weight, P > 0.05 for +/+ versus −/− animals of the same sex on each diet. (C) Body composition after 10 weeks on normal, high-fat, and high-protein diets. Males, **, P < 0.001 high-fat versus normal and high-protein diets; females, *, _P_ < 0.05 high-fat versus normal and high-protein diets. _P_ > 0.05 for +/+ versus −/− animals within each diet.
Similar articles
- Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor.
Sun Y, Wang P, Zheng H, Smith RG. Sun Y, et al. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4679-84. doi: 10.1073/pnas.0305930101. Epub 2004 Mar 18. Proc Natl Acad Sci U S A. 2004. PMID: 15070777 Free PMC article. - Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference.
Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Wortley KE, et al. Proc Natl Acad Sci U S A. 2004 May 25;101(21):8227-32. doi: 10.1073/pnas.0402763101. Epub 2004 May 17. Proc Natl Acad Sci U S A. 2004. PMID: 15148384 Free PMC article. - Mice lacking ghrelin receptors resist the development of diet-induced obesity.
Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Zigman JM, et al. J Clin Invest. 2005 Dec;115(12):3564-72. doi: 10.1172/JCI26002. J Clin Invest. 2005. PMID: 16322794 Free PMC article. - Clinical endocrinology and metabolism. Ghrelin, a novel growth-hormone-releasing and appetite-stimulating peptide from stomach.
Kojima M, Hosoda H, Kangawa K. Kojima M, et al. Best Pract Res Clin Endocrinol Metab. 2004 Dec;18(4):517-30. doi: 10.1016/j.beem.2004.07.001. Best Pract Res Clin Endocrinol Metab. 2004. PMID: 15533773 Review. - Drug insight: The functions of ghrelin and its potential as a multitherapeutic hormone.
Kojima M, Kangawa K. Kojima M, et al. Nat Clin Pract Endocrinol Metab. 2006 Feb;2(2):80-8. doi: 10.1038/ncpendmet0080. Nat Clin Pract Endocrinol Metab. 2006. PMID: 16932262 Review.
Cited by
- Central manipulation of dopamine receptors attenuates the orexigenic action of ghrelin.
Romero-Picó A, Novelle MG, Folgueira C, López M, Nogueiras R, Diéguez C. Romero-Picó A, et al. Psychopharmacology (Berl). 2013 Sep;229(2):275-83. doi: 10.1007/s00213-013-3096-7. Epub 2013 Apr 28. Psychopharmacology (Berl). 2013. PMID: 23624808 - Activation of somatostatin 2 receptors in the brain and the periphery induces opposite changes in circulating ghrelin levels: functional implications.
Stengel A, Taché Y. Stengel A, et al. Front Endocrinol (Lausanne). 2013 Jan 11;3:178. doi: 10.3389/fendo.2012.00178. eCollection 2012. Front Endocrinol (Lausanne). 2013. PMID: 23335913 Free PMC article. - Is gastrectomy-induced high turnover of bone with hyperosteoidosis and increase of mineralization a typical osteomalacia?
Ueyama T, Yamamoto Y, Ueda K, Yajima A, Maeda Y, Yamashita Y, Ito T, Tsuruo Y, Ichinose M. Ueyama T, et al. PLoS One. 2013 Jun 11;8(6):e65685. doi: 10.1371/journal.pone.0065685. Print 2013. PLoS One. 2013. PMID: 23776526 Free PMC article. - Ghrelin treatment reverses the reduction in weight gain and body fat in gastrectomised mice.
Dornonville de la Cour C, Lindqvist A, Egecioglu E, Tung YC, Surve V, Ohlsson C, Jansson JO, Erlanson-Albertsson C, Dickson SL, Håkanson R. Dornonville de la Cour C, et al. Gut. 2005 Jul;54(7):907-13. doi: 10.1136/gut.2004.058578. Epub 2005 Apr 21. Gut. 2005. PMID: 15849166 Free PMC article. - Ghrelin's Relationship to Blood Glucose.
Mani BK, Shankar K, Zigman JM. Mani BK, et al. Endocrinology. 2019 May 1;160(5):1247-1261. doi: 10.1210/en.2019-00074. Endocrinology. 2019. PMID: 30874792 Free PMC article. Review.
References
- Bagnasco, M., P. S. Kalra, and S. P. Kalra. 2002. Ghrelin and leptin pulse discharge in fed and fasted rats. Endocrinology 143:726-729. - PubMed
- Bailey, A. R. T., R. G. Smith, and G. Leng. 1998. The non-peptide growth hormone secretagogue, MK-0677, activates hypothalamic neurones in vivo. J. Neuroendocrinol. 10:111-118. - PubMed
- Beck, B., N. Musse, and A. Stricker-Krongrad. 2002. Ghrelin, macronutrient intake and dietary preferences in long-evans rats. Biochem. Biophys. Res. Commun. 292:1031-1035. - PubMed
- Chapman, I. M., M. A. Bach, C. Van, E., M. Farmer, D. A. Krupa, A. M. Taylor, L. M. Schilling, K. Y. Cole, E. H. Skiles, S. S. Pezzoli, M. L. Hartman, J. D. Veldhuis, G. J. Gormley, and M. O. Thorner. 1996. Stimulation of the growth hormone (GH)-insulin-like growth factor-I axis by daily oral administration of a GH secretagogue (MK-0677) in healthy elderly subjects. J. Clin. Endocrinol. Metab. 81:4249-4257. - PubMed
- Cowley, M., R. G. Smith, S. Diano, M. Tschop, N. Pronchuk, K. Grove, C. Strasburger, M. Bidlingmaier, M. Esterman, M. Heiman, L. Garcia-Segura, E. Nillni, P. Mendez, M. Low, P. Sotonyi, J. Friedman, H. Liu, S. Pinto, W. Colmers, R. Cone, and T. Horvath. 2003. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37:649-661. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials