The death domain kinase RIP1 is essential for tumor necrosis factor alpha signaling to p38 mitogen-activated protein kinase - PubMed (original) (raw)
The death domain kinase RIP1 is essential for tumor necrosis factor alpha signaling to p38 mitogen-activated protein kinase
Thomas H Lee et al. Mol Cell Biol. 2003 Nov.
Abstract
The cytokine tumor necrosis factor alpha (TNF-alpha) stimulates the NF-kappaB, SAPK/JNK, and p38 mitogen-activated protein (MAP) kinase pathways by recruiting RIP1 and TRAF2 proteins to the tumor necrosis factor receptor 1 (TNFR1). Genetic studies have revealed that RIP1 links the TNFR1 to the IkappaB kinase (IKK) complex, whereas TRAF2 couples the TNFR1 to the SAPK/JNK cascade. In transfection studies, RIP1 and TRAF2 stimulate p38 MAP kinase activation, and dominant-negative forms of RIP1 and TRAF2 inhibit TNF-alpha-induced p38 MAP kinase activation. We found TNF-alpha-induced p38 MAP kinase activation and interleukin-6 (IL-6) production impaired in rip1(-/-) murine embryonic fibroblasts (MEF) but unaffected in traf2(-/-) MEF. Yet, both rip1(-/-) and traf2(-/-) MEF exhibit a normal p38 MAP kinase response to inducers of osmotic shock or IL-1alpha. Thus, RIP1 is a specific mediator of the p38 MAP kinase response to TNF-alpha. These studies suggest that TNF-alpha-induced activation of p38 MAP kinase and SAPK/JNK pathways bifurcate at the level of RIP1 and TRAF2. Moreover, endogenous RIP1 associates with the MAP kinase kinase kinase (MAP3K) MEKK3 in TNF-alpha-treated cells, and decreased TNF-alpha-induced p38 MAP kinase activation is observed in Mekk3(-/-) cells. Taken together, these studies suggest a mechanism whereby RIP1 may mediate the p38 MAP kinase response to TNF-alpha, by recruiting the MAP3K MEKK3.
Figures
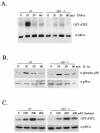
FIG. 1.
TNF-α-induced p38 MAPK activity is impaired in _rip1_−/− cells. (A) Wild-type and _rip_−/− MEF were grown to confluence on 10-cm-diameter plates and treated with 10 ng of murine TNF-α/ml for 0, 10, 30, and 60 min. Cells were subsequently lysed and immunoprecipitated with anti-p38α Ab (a gift from R. Davis), and the kinase activity of p38 MAPK was measured by an in vitro kinase assay with GST-ATF2 as substrate. The p38α level in each cell type was determined by immunoblotting with anti-p38α (Santa Cruz). (B) Normal IL-1α-induced p38 MAPK activity in _rip_−/− cells. Wild-type and _rip_−/− MEF were treated with 10 ng of IL-1α/ml for various times (0, 10, 30, and 60 min), and the p38 MAPK activity was measured by immunoblotting with an anti-phospho-p38 Ab (Cell Signaling Technology catalog no. 9211S). (C) Normal sorbitol-induced p38 MAPK activity in _rip_−/− cells. Wild-type and _rip_−/− MEF were treated with 0, 100, 200, and 400 mM sorbitol for 15 min, and the kinase activity of p38 was measured by in vitro kinase assay with GST-ATF2 as substrate.
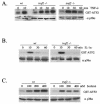
FIG. 2.
TNF-α-induced p38 MAPK activity is unaffected in _traf2_−/− cells. (A) Wild-type and _traf2_−/− MEF were grown to confluence on 10-cm-diameter plates and treated with 10 ng of TNF-α/ml for 0, 10, 30, and 60 min. Cells were lysed and immunoprecipitated with anti-p38α Ab (a gift from R. Davis), and the kinase activity of p38 MAPK was measured by an in vitro kinase assay using GST-ATF2 as substrate. The p38α level in each cell type was determined by immunoblotting with anti-p38α (Santa Cruz). (B) IL-1α-induced p38 MAPK activity is unaffected in _traf2_−/− cells. Wild-type and _traf2_−/− MEF were treated with 10 ng of IL-1α/ml and measured for p38 activity with anti-phospho-p38 Ab. (C) Sorbitol-induced p38 MAPK activity is unaffected in _traf2_−/− cells. Wild-type and _traf2_−/− MEF were also treated with 0, 100, 200, and 400 mM sorbitol for 15 min, and p38 MAPK activity was measured by an in vitro kinase assay with GST-ATF2 as substrate.
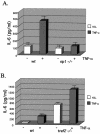
FIG. 3.
Decreased TNF-α-induced IL-6 production in _rip1_−/− cells. (A) Wild-type and _rip1_−/− MEF were plated at 3 × 104 cells per well on 24-well plates and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit (PharMingen catalog no. 2653KI). (B) TNF-α-induced IL-6 production in _traf2_−/− cells. Wild-type and _traf2_−/− cells were plated on 24-well plates at 3 × 104 cells per well and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit. The amount of IL-6 is presented as the mean ± standard deviation of triplicate observations. Similar data were obtained in six independent experiments with six independent _rip_−/− MEF lines and five _traf2_−/− MEF lines. (C) Decreased TNF-α-induced IL-6 mRNA in _rip1_−/− MEF. Wild-type and _rip1_−/− MEF were left untreated or treated with TNF-α (10 ng/ml) for 2 and 24 h. The amount of IL-6 mRNA and GAPDH mRNA was measured by RNase protection assay. The protected RNA was detected by autoradiography after denaturing polyacrylamide gel electrophoresis and was quantitated by PhosphorImager analysis. For clarity, exposure time varied for autoradiography for IL-6 and GAPDH mRNAs. Similar data were obtained in three separate experiments. (D) TNF-α-induced p38 MAPK activation in _rip1_−/− MEF infected with RIP1 retrovirus. _rip1_−/− MEF were infected with vector alone (MSCV) or with a RIP1 retrovirus [_rip1_−/− (rip1)]. Wild-type, _rip1_−/−, _rip1_−/− (MSCV), and _rip1_−/− (rip1) cells were left untreated or stimulated with TNF-α for the time periods indicated, and p38 MAPK activity was measured by immunoblotting with an anti-phospho-p38 Ab. The RIP1 and p38α expression was determined by immunoblotting with anti-RIP and anti-p38α Abs. The percentage of green fluorescent protein-positive cells in the infected populations was determined by flow cytometry. Results from one of three independent infection experiments are shown. (E) TNF-α-induced IL-6 production is increased in _rip1_−/− MEF infected with a RIP1 retrovirus. Wild-type, _rip1_−/−, and _rip1_−/− MEF infected with vector (MSCV) or with a RIP1 retrovirus [_rip1_−/− (rip1)] were plated at 3 × 104 cells per well on 24-well plates and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit. The amount of IL-6 is presented as the mean ± standard deviation of triplicate observations.
FIG. 3.
Decreased TNF-α-induced IL-6 production in _rip1_−/− cells. (A) Wild-type and _rip1_−/− MEF were plated at 3 × 104 cells per well on 24-well plates and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit (PharMingen catalog no. 2653KI). (B) TNF-α-induced IL-6 production in _traf2_−/− cells. Wild-type and _traf2_−/− cells were plated on 24-well plates at 3 × 104 cells per well and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit. The amount of IL-6 is presented as the mean ± standard deviation of triplicate observations. Similar data were obtained in six independent experiments with six independent _rip_−/− MEF lines and five _traf2_−/− MEF lines. (C) Decreased TNF-α-induced IL-6 mRNA in _rip1_−/− MEF. Wild-type and _rip1_−/− MEF were left untreated or treated with TNF-α (10 ng/ml) for 2 and 24 h. The amount of IL-6 mRNA and GAPDH mRNA was measured by RNase protection assay. The protected RNA was detected by autoradiography after denaturing polyacrylamide gel electrophoresis and was quantitated by PhosphorImager analysis. For clarity, exposure time varied for autoradiography for IL-6 and GAPDH mRNAs. Similar data were obtained in three separate experiments. (D) TNF-α-induced p38 MAPK activation in _rip1_−/− MEF infected with RIP1 retrovirus. _rip1_−/− MEF were infected with vector alone (MSCV) or with a RIP1 retrovirus [_rip1_−/− (rip1)]. Wild-type, _rip1_−/−, _rip1_−/− (MSCV), and _rip1_−/− (rip1) cells were left untreated or stimulated with TNF-α for the time periods indicated, and p38 MAPK activity was measured by immunoblotting with an anti-phospho-p38 Ab. The RIP1 and p38α expression was determined by immunoblotting with anti-RIP and anti-p38α Abs. The percentage of green fluorescent protein-positive cells in the infected populations was determined by flow cytometry. Results from one of three independent infection experiments are shown. (E) TNF-α-induced IL-6 production is increased in _rip1_−/− MEF infected with a RIP1 retrovirus. Wild-type, _rip1_−/−, and _rip1_−/− MEF infected with vector (MSCV) or with a RIP1 retrovirus [_rip1_−/− (rip1)] were plated at 3 × 104 cells per well on 24-well plates and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit. The amount of IL-6 is presented as the mean ± standard deviation of triplicate observations.
FIG. 3.
Decreased TNF-α-induced IL-6 production in _rip1_−/− cells. (A) Wild-type and _rip1_−/− MEF were plated at 3 × 104 cells per well on 24-well plates and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit (PharMingen catalog no. 2653KI). (B) TNF-α-induced IL-6 production in _traf2_−/− cells. Wild-type and _traf2_−/− cells were plated on 24-well plates at 3 × 104 cells per well and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit. The amount of IL-6 is presented as the mean ± standard deviation of triplicate observations. Similar data were obtained in six independent experiments with six independent _rip_−/− MEF lines and five _traf2_−/− MEF lines. (C) Decreased TNF-α-induced IL-6 mRNA in _rip1_−/− MEF. Wild-type and _rip1_−/− MEF were left untreated or treated with TNF-α (10 ng/ml) for 2 and 24 h. The amount of IL-6 mRNA and GAPDH mRNA was measured by RNase protection assay. The protected RNA was detected by autoradiography after denaturing polyacrylamide gel electrophoresis and was quantitated by PhosphorImager analysis. For clarity, exposure time varied for autoradiography for IL-6 and GAPDH mRNAs. Similar data were obtained in three separate experiments. (D) TNF-α-induced p38 MAPK activation in _rip1_−/− MEF infected with RIP1 retrovirus. _rip1_−/− MEF were infected with vector alone (MSCV) or with a RIP1 retrovirus [_rip1_−/− (rip1)]. Wild-type, _rip1_−/−, _rip1_−/− (MSCV), and _rip1_−/− (rip1) cells were left untreated or stimulated with TNF-α for the time periods indicated, and p38 MAPK activity was measured by immunoblotting with an anti-phospho-p38 Ab. The RIP1 and p38α expression was determined by immunoblotting with anti-RIP and anti-p38α Abs. The percentage of green fluorescent protein-positive cells in the infected populations was determined by flow cytometry. Results from one of three independent infection experiments are shown. (E) TNF-α-induced IL-6 production is increased in _rip1_−/− MEF infected with a RIP1 retrovirus. Wild-type, _rip1_−/−, and _rip1_−/− MEF infected with vector (MSCV) or with a RIP1 retrovirus [_rip1_−/− (rip1)] were plated at 3 × 104 cells per well on 24-well plates and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit. The amount of IL-6 is presented as the mean ± standard deviation of triplicate observations.
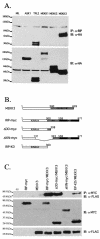
FIG. 4.
RIP1 associates with the MAP3K MEKK3 in TNF-α-stimulated cells. (A) RIP1 interacts with MEKK3 and Tpl2 kinases. 293T cells were left untransfected (NIL) or transfected with HA-ASK1, HA-Tpl2, HA-MEKK1, HA-MEKK2, or HA-MEKK3. The cells were lysed and immunoprecipitated with anti-RIP Ab and immunoblotted with anti-HA Ab. Expression of transfected constructs was confirmed by immunoblotting with anti-HA. (B) Schematic representation of MEKK3, RIP1, and the RIP1 deletion constructs used to map interactions. (C) The kinase domain of RIP1 binds MEKK3. 293T cells were transfected with FLAG-tagged MEKK3 and various MYC-tagged RIP1 deletion constructs. The transfected cells were immunoprecipitated with anti-MYC Ab, and the associated MEKK3 proteins were detected by immunoblotting with anti-FLAG Ab. Expression of various RIP1-MYC deletion constructs was determined by immunoblotting with anti-MYC Ab. (D) Kinase-inactive versions of RIP1 fail to interact with MEKK3. 293T cells were transfected with a FLAG-tagged MEKK3 and either wild-type, MYC-tagged RIP1, or MYC-tagged kinase-inactive versions of RIP1 (RIP1D138N and RIP1K45R). Cells were lysed and immunoprecipitated with anti-MYC Ab, and MEKK3 protein was detected by immunoblotting with an anti-FLAG Ab. Expression levels of various constructs were determined by immunoblotting with anti-MYC and anti-FLAG Abs. (E) Endogenous RIP1-MEKK3 interaction is enhanced in TNF-α-stimulated cells. 293T cells were left untreated or treated with 100 ng of human TNF-α/ml for the time periods indicated and then immunoprecipitated with anti-MEKK3 Ab. RIP1 proteins were detected by immunoblotting with anti-RIP Ab (PharMingen). (F) TNF-α-induced p38 MAPK activation is impaired in _Mekk3_−/− MEF. Two wild-type and three _Mekk3_−/− MEF lines were left untreated or stimulated with TNF-α for the time periods indicated, and p38 MAPK activity was measured by immunoblotting with an anti-phospho-p38 Ab. The RIP1 and p38α expression was determined by immunoblotting with anti-RIP and anti-p38α Abs.
FIG. 4.
RIP1 associates with the MAP3K MEKK3 in TNF-α-stimulated cells. (A) RIP1 interacts with MEKK3 and Tpl2 kinases. 293T cells were left untransfected (NIL) or transfected with HA-ASK1, HA-Tpl2, HA-MEKK1, HA-MEKK2, or HA-MEKK3. The cells were lysed and immunoprecipitated with anti-RIP Ab and immunoblotted with anti-HA Ab. Expression of transfected constructs was confirmed by immunoblotting with anti-HA. (B) Schematic representation of MEKK3, RIP1, and the RIP1 deletion constructs used to map interactions. (C) The kinase domain of RIP1 binds MEKK3. 293T cells were transfected with FLAG-tagged MEKK3 and various MYC-tagged RIP1 deletion constructs. The transfected cells were immunoprecipitated with anti-MYC Ab, and the associated MEKK3 proteins were detected by immunoblotting with anti-FLAG Ab. Expression of various RIP1-MYC deletion constructs was determined by immunoblotting with anti-MYC Ab. (D) Kinase-inactive versions of RIP1 fail to interact with MEKK3. 293T cells were transfected with a FLAG-tagged MEKK3 and either wild-type, MYC-tagged RIP1, or MYC-tagged kinase-inactive versions of RIP1 (RIP1D138N and RIP1K45R). Cells were lysed and immunoprecipitated with anti-MYC Ab, and MEKK3 protein was detected by immunoblotting with an anti-FLAG Ab. Expression levels of various constructs were determined by immunoblotting with anti-MYC and anti-FLAG Abs. (E) Endogenous RIP1-MEKK3 interaction is enhanced in TNF-α-stimulated cells. 293T cells were left untreated or treated with 100 ng of human TNF-α/ml for the time periods indicated and then immunoprecipitated with anti-MEKK3 Ab. RIP1 proteins were detected by immunoblotting with anti-RIP Ab (PharMingen). (F) TNF-α-induced p38 MAPK activation is impaired in _Mekk3_−/− MEF. Two wild-type and three _Mekk3_−/− MEF lines were left untreated or stimulated with TNF-α for the time periods indicated, and p38 MAPK activity was measured by immunoblotting with an anti-phospho-p38 Ab. The RIP1 and p38α expression was determined by immunoblotting with anti-RIP and anti-p38α Abs.
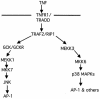
FIG. 5.
Proposed model for JNK and p38 MAPK activation in response to the cytokine TNF-α. TNF-α-induced JNK activation is mediated by TRAF2-dependent recruitment of GCK-GCKR and subsequent activation of the MAP3K MEKK1 (6), whereas TNF-α-induced p38 MAPK activation may be mediated by the RIP1-dependent recruitment of the MAP3K MEKK3 and subsequent activation of MKK3-MKK6. The precise mechanism(s) of RIP1-mediated activation of MEKK3 remains unclear.
Similar articles
- The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2.
Lee TH, Shank J, Cusson N, Kelliher MA. Lee TH, et al. J Biol Chem. 2004 Aug 6;279(32):33185-91. doi: 10.1074/jbc.M404206200. Epub 2004 Jun 1. J Biol Chem. 2004. PMID: 15175328 - RIP1-mediated AIP1 phosphorylation at a 14-3-3-binding site is critical for tumor necrosis factor-induced ASK1-JNK/p38 activation.
Zhang H, Zhang H, Lin Y, Li J, Pober JS, Min W. Zhang H, et al. J Biol Chem. 2007 May 18;282(20):14788-96. doi: 10.1074/jbc.M701148200. Epub 2007 Mar 27. J Biol Chem. 2007. PMID: 17389591 - Tumor necrosis factor alpha-induced activation of c-jun N-terminal kinase is mediated by TRAF2.
Reinhard C, Shamoon B, Shyamala V, Williams LT. Reinhard C, et al. EMBO J. 1997 Mar 3;16(5):1080-92. doi: 10.1093/emboj/16.5.1080. EMBO J. 1997. PMID: 9118946 Free PMC article. - TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death.
Borghi A, Verstrepen L, Beyaert R. Borghi A, et al. Biochem Pharmacol. 2016 Sep 15;116:1-10. doi: 10.1016/j.bcp.2016.03.009. Epub 2016 Mar 16. Biochem Pharmacol. 2016. PMID: 26993379 Review. - Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice.
Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. Matsuzawa A, et al. Antioxid Redox Signal. 2002 Jun;4(3):415-25. doi: 10.1089/15230860260196218. Antioxid Redox Signal. 2002. PMID: 12215209 Review.
Cited by
- Restoration of NF-kappaB activation by tumor necrosis factor alpha receptor complex-targeted MEKK3 in receptor-interacting protein-deficient cells.
Blonska M, You Y, Geleziunas R, Lin X. Blonska M, et al. Mol Cell Biol. 2004 Dec;24(24):10757-65. doi: 10.1128/MCB.24.24.10757-10765.2004. Mol Cell Biol. 2004. PMID: 15572679 Free PMC article. - Arrestins in apoptosis.
Kook S, Gurevich VV, Gurevich EV. Kook S, et al. Handb Exp Pharmacol. 2014;219:309-39. doi: 10.1007/978-3-642-41199-1_16. Handb Exp Pharmacol. 2014. PMID: 24292837 Free PMC article. - The Receptor-interacting Serine/Threonine Protein Kinase 1 (RIPK1) Regulates Progranulin Levels.
Mason AR, Elia LP, Finkbeiner S. Mason AR, et al. J Biol Chem. 2017 Feb 24;292(8):3262-3272. doi: 10.1074/jbc.M116.752006. Epub 2017 Jan 9. J Biol Chem. 2017. PMID: 28069809 Free PMC article. - Proteome Analysis of Swine Macrophages after Infection with Two Genotype II African Swine Fever Isolates of Different Pathogenicity.
Wöhnke E, Cackett G, Werner F, Blome S, Mettenleiter TC, Karger A. Wöhnke E, et al. Viruses. 2022 Sep 28;14(10):2140. doi: 10.3390/v14102140. Viruses. 2022. PMID: 36298696 Free PMC article. - Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation.
Cuchet-Lourenço D, Eletto D, Wu C, Plagnol V, Papapietro O, Curtis J, Ceron-Gutierrez L, Bacon CM, Hackett S, Alsaleem B, Maes M, Gaspar M, Alisaac A, Goss E, AlIdrissi E, Siegmund D, Wajant H, Kumararatne D, AlZahrani MS, Arkwright PD, Abinun M, Doffinger R, Nejentsev S. Cuchet-Lourenço D, et al. Science. 2018 Aug 24;361(6404):810-813. doi: 10.1126/science.aar2641. Epub 2018 Jul 19. Science. 2018. PMID: 30026316 Free PMC article.
References
- Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. Nebreda. 2000. Essential role of p38 alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6:109-116. - PubMed
- Bagrodia, S., B. Derijard, R. J. Davis, and R. A. Cerione. 1995. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 270:27995-27998. - PubMed
- Blank, J. L., et al. 1996. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J. Biol. Chem. 271:5361-5368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous