Receptor internalization in yeast requires the Tor2-Rho1 signaling pathway - PubMed (original) (raw)
Receptor internalization in yeast requires the Tor2-Rho1 signaling pathway
Amy K A deHart et al. Mol Biol Cell. 2003 Nov.
Abstract
Efficient internalization of proteins from the cell surface is essential for regulating cell growth and differentiation. In a screen for yeast mutants defective in ligand-stimulated internalization of the alpha-factor receptor, we identified a mutant allele of TOR2, tor2G2128R. Tor proteins are known to function in translation initiation and nutrient sensing and are required for cell cycle progression through G1. Yeast Tor2 has an additional role in regulating the integrity of the cell wall by activating the Rho1 guanine nucleotide exchange factor Rom2. The endocytic defect in tor2G2128R cells is due to disruption of this Tor2 unique function. Other proteins important for cell integrity, Rom2 and the cell integrity sensor Wsc1, are also required for efficient endocytosis. A rho1 mutant specifically defective in activation of the glucan synthase Fks1/2 does not internalize alpha-factor efficiently, and fks1Delta cells exhibit a similar phenotype. Removal of the cell wall does not inhibit internalization, suggesting that the function of Rho1 and Fks1 in endocytosis is not through cell wall synthesis or structural integrity. These findings reveal a novel function for the Tor2-Rho1 pathway in controlling endocytosis in yeast, a function that is mediated in part through the plasma membrane protein Fks1.
Figures
Figure 1.
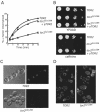
Tor2 is required for receptor-mediated and fluid phase internalization. (A) Internalization of 35S-α-factor was measured at 30°C after growth of cells in YPUAD at 24°C. Wild-type (LHY3865, ♦); tor2G2128R (LHY3863, □); tor2G2128R with p_TOR2_ (LHY4306, ▴). These same strains were used for the experiments shown in all parts of the figure. (B) Wild-type, tor2G2128R, or tor2G2128R cells carrying centromere-based p_TOR2_ were grown on YPUAD or YPUAD + 6 mM caffeine plates at 30°C. (C) Lucifer yellow localization was assayed in wild-type and tor2G2128R cells. Images were taken using differential interference contrast and fluorescence optics. (D) The actin cytoskeleton was stained in wild-type cells and in tor2G2128R cells. Cells were shifted to 30°C for 3 h before fixation and incubation with FITC-phalloidin. In cells with emerging buds, 29.4% of wild-type cells showed poor polarization of the cytoskeleton (see MATERIALS AND METHODS), compared with 48.4% in tor2G2128R cells.
Figure 2.
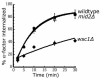
The unique function of Tor2 is required for its role in receptor internalization. Internalization of 35S-α-factor was measured at 30°C after growth of cells in YPUAD at 24°C. (A) Wild-type (LHY2632, ♦), _rom1_Δ (LHY3741, ▴), _rom2_Δ (LHY3742, ▪), and _tus1_Δ (LHY3749, ⋄). (B) The same strains assayed in C were tested for growth on YPUAD or YPUAD + 6 mM caffeine plates at 30°C. (C) Wild-type (LHY3877, ♦), tor2G2128R (LHY3878, ▪), _sac7_Δ (LHY3879, Δ), and _tor2G2128R sac7_Δ (LHY3880, ⋄). (D) Wild-type (LHY3865; ⋄, ♦) and tor2G2128R (LHY3863; □, ▪) cells were assayed either in YPUAD (open symbol) or YPUAD + 0.005% SDS (closed symbol).
Figure 3.
Cell wall integrity sensor Wsc1 is required for internalization. Wild-type (LHY2632, ▵), _wsc1_Δ (LHY3946, ♦), and _mid2_Δ (LHY4143, ▪) cells were assayed for internalization of 35S-α-factor at 30°C after growth in YPUAD at 24°C.
Figure 4.
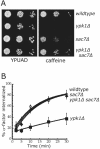
Ypk1 regulates receptor internalization through Rom2-Rho1. (A) Wild-type (LHY4388), _ypk1_Δ (LHY4387), _sac7_Δ (LHY4389), and _ypk1_Δ _sac7_Δ (LHY4390) cells were grown on YPUAD or YPUAD + 6 mM caffeine plates at 30°C. (B) The same strains were assayed for internalization of 35S-α-factor at 30°C after growth of cells in YPUAD at 24°C. Wild type (♦), _ypk1_Δ (▪), _sac7_Δ (Δ), and _ypk1_Δ _sac7_Δ (⋄).
Figure 5.
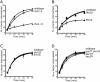
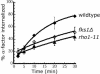
Fks1 is a Rho1 effector required for internalization. Internalization of 35S-α-factor was measured at the indicated temperature after growth of cells in YPUAD at 24°C. (A) Wild-type (LHY3604, ♦), rho1-2 (LHY3602, □), and rho1-11 (LHY3603, ▵) cells were assayed at 37°C. (B) Wild-type (LHY4515, ♦), _fks1_Δ (LHY4477, ▴), and _fks2_Δ (LHY4514, ▪) cells were assayed at 30°C. (C) Wild-type (LHY2632, ♦), _skn7_Δ (LHY3746, ⋄), and _bni1_Δ (LHY3421, X) cells were assayed at 30°C. (D) Wild-type (LHY4172, ♦), _bnr1_Δ bni1ts (LHY4171, ▪), pkc1t s (LHY2817, □), and sec3ts (LHY3745, ▵) cells were assayed at 37°C.
Figure 6.
Cell wall damage does not inhibit internalization. Wild-type (LHY2632, ♦), _fks1_Δ (LHY3736, ⋄), and rho1-11 (LHY3603, ▴) cells were grown in YPUAD at 24°C, converted to spheroplasts, incubated for 20-30 min in recovery buffer, and then assayed for internalization of 35S-α-factor at 30°C.
Figure 7.
Cell integrity pathway components involved in regulating receptor internalization. A schematic diagram of the proteins of the cell integrity pathway that are involved in the regulation of endocytosis. 1) Proteins required for the activation of Rho1 (Tor2, Wsc1, and Rom2) are required for efficient internalization. 2) The endocytic function of Ypk1 is mediated in part by this pathway. Our results are consistent with a role for Ypk1 upstream of Rho1. Ypk1 is likely to be activated by Tor2-dependent phosphorylation (see DISCUSSION); however, the target of Ypk1 action is not known. 3) The Rho1 effector Fks1 is required for endocytosis, perhaps by influencing the localization of components of the endocytic machinery or by affecting localization of Rho1 itself, which may be necessary for activation of an unidentified Rho1 effector and/or control of PI metabolism.
Similar articles
- Cell wall integrity modulates RHO1 activity via the exchange factor ROM2.
Bickle M, Delley PA, Schmidt A, Hall MN. Bickle M, et al. EMBO J. 1998 Apr 15;17(8):2235-45. doi: 10.1093/emboj/17.8.2235. EMBO J. 1998. PMID: 9545237 Free PMC article. - The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2.
Schmidt A, Bickle M, Beck T, Hall MN. Schmidt A, et al. Cell. 1997 Feb 21;88(4):531-42. doi: 10.1016/s0092-8674(00)81893-0. Cell. 1997. PMID: 9038344 - Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1.
Philip B, Levin DE. Philip B, et al. Mol Cell Biol. 2001 Jan;21(1):271-80. doi: 10.1128/MCB.21.1.271-280.2001. Mol Cell Biol. 2001. PMID: 11113201 Free PMC article. - Insight into Tor2, a budding yeast microdomain protein.
Bartlett K, Kim K. Bartlett K, et al. Eur J Cell Biol. 2014 Mar;93(3):87-97. doi: 10.1016/j.ejcb.2014.01.004. Epub 2014 Feb 14. Eur J Cell Biol. 2014. PMID: 24629393 Review. - Cell wall integrity signaling in Saccharomyces cerevisiae.
Levin DE. Levin DE. Microbiol Mol Biol Rev. 2005 Jun;69(2):262-91. doi: 10.1128/MMBR.69.2.262-291.2005. Microbiol Mol Biol Rev. 2005. PMID: 15944456 Free PMC article. Review.
Cited by
- Efficient Tor signaling requires a functional class C Vps protein complex in Saccharomyces cerevisiae.
Zurita-Martinez SA, Puria R, Pan X, Boeke JD, Cardenas ME. Zurita-Martinez SA, et al. Genetics. 2007 Aug;176(4):2139-50. doi: 10.1534/genetics.107.072835. Epub 2007 Jun 11. Genetics. 2007. PMID: 17565946 Free PMC article. - Inactivation of Tor proteins affects the dynamics of endocytic proteins in early stage of endocytosis.
Tenay B, Kimberlin E, Williams M, Denise J, Fakilahyel J, Kim K. Tenay B, et al. J Biosci. 2013 Jun;38(2):351-61. doi: 10.1007/s12038-013-9326-7. J Biosci. 2013. PMID: 23660670 - Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization.
Kamada Y, Fujioka Y, Suzuki NN, Inagaki F, Wullschleger S, Loewith R, Hall MN, Ohsumi Y. Kamada Y, et al. Mol Cell Biol. 2005 Aug;25(16):7239-48. doi: 10.1128/MCB.25.16.7239-7248.2005. Mol Cell Biol. 2005. PMID: 16055732 Free PMC article. - Integrins in disguise - mechanosensors in Saccharomyces cerevisiae as functional integrin analogues.
Elhasi T, Blomberg A. Elhasi T, et al. Microb Cell. 2019 Jul 15;6(8):335-355. doi: 10.15698/mic2019.08.686. Microb Cell. 2019. PMID: 31404395 Free PMC article. Review. - Calcineurin determines toxic versus beneficial responses to α-synuclein.
Caraveo G, Auluck PK, Whitesell L, Chung CY, Baru V, Mosharov EV, Yan X, Ben-Johny M, Soste M, Picotti P, Kim H, Caldwell KA, Caldwell GA, Sulzer D, Yue DT, Lindquist S. Caraveo G, et al. Proc Natl Acad Sci U S A. 2014 Aug 26;111(34):E3544-52. doi: 10.1073/pnas.1413201111. Epub 2014 Aug 13. Proc Natl Acad Sci U S A. 2014. PMID: 25122673 Free PMC article.
References
- Alberts, A.S., Bouquin, N., Johnston, L.H., and Treisman, R. (1998). Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J. Biol. Chem. 273, 8616-8622. - PubMed
- Audhya, A., and Emr, S.D. (2002). Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2, 593-605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases