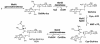Mycothiol is essential for growth of Mycobacterium tuberculosis Erdman - PubMed (original) (raw)
Mycothiol is essential for growth of Mycobacterium tuberculosis Erdman
Dipti Sareen et al. J Bacteriol. 2003 Nov.
Abstract
Mycothiol (MSH) is the major low-molecular-mass thiol in mycobacteria and is associated with the protection of Mycobacterium tuberculosis from toxic oxidants and antibiotics. The biosynthesis of MSH is a multistep process, with the enzymatic reaction designated MshC being the ligase step in MSH production. A targeted disruption of the native mshC gene in M. tuberculosis Erdman produced no viable clones possessing either a disrupted mshC gene or reduced levels of MSH. However, when a second copy of the mshC gene was incorporated into the chromosome prior to the targeted disruption, multiple clones having the native gene disrupted and the second copy of mshC intact were obtained. These clones produced normal levels of MSH. These results demonstrate that the mshC gene and, more generally, the production of MSH are essential for the growth of M. tuberculosis Erdman under laboratory conditions.
Figures
FIG. 1.
Key enzymes in the biosynthesis of MSH in M. tuberculosis include the glycosyltransferase (MshA, encoded by Rv0486), the GlcNAc-Ins deacetylase (MshB, encoded by Rv1170), the ATP-dependent Cys:GlcN-Ins ligase (MshC, encoded by Rv2130c), and the acetyl-CoA (CoASAc):Cys-GlcN-Ins acetyltransferase (MSH synthase; MshD, encoded by Rv0819).
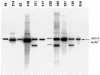
FIG. 2.
Southern blot of chromosomal DNA digested with _Nco_I and probed with a 500-bp PCR fragment containing residues 195 to 708 from within the mshC gene. Erd, M. tuberculosis Erdman. Clones 59, 214, 63, 318, 317, and 331 have a single copy of mshC identical to that of the wild-type strain. Clones 151, 226, 182, and 135 have the native mshC intact and a component of the knockout substrate incorporated by nonhomologous recombination.
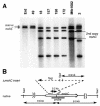
FIG. 3.
(A) Southern blot of chromosomal DNA digested with _Sac_I and probed with a 500-bp fragment containing residues 195 to 708 from within the mshC gene. Erd, M. tuberculosis Erdman. Clone 49 is derived from wild-type M. tuberculosis Erdman, and clones 3, 18, 157, 158, and 172 are derived from Mtb1682 (2X-mshC). (B) Diagram showing location of _Sac_I sites within the M. tuberculosis H37Rv genome near mshC and within the insert of the mshC knockout.
Similar articles
- The mshA gene encoding the glycosyltransferase of mycothiol biosynthesis is essential in Mycobacterium tuberculosis Erdman.
Buchmeier N, Fahey RC. Buchmeier N, et al. FEMS Microbiol Lett. 2006 Nov;264(1):74-9. doi: 10.1111/j.1574-6968.2006.00441.x. FEMS Microbiol Lett. 2006. PMID: 17020551 - Mycothiol biochemistry.
Newton GL, Fahey RC. Newton GL, et al. Arch Microbiol. 2002 Dec;178(6):388-94. doi: 10.1007/s00203-002-0469-4. Epub 2002 Sep 3. Arch Microbiol. 2002. PMID: 12420157 Review. - Association of mycothiol with protection of Mycobacterium tuberculosis from toxic oxidants and antibiotics.
Buchmeier NA, Newton GL, Koledin T, Fahey RC. Buchmeier NA, et al. Mol Microbiol. 2003 Mar;47(6):1723-32. doi: 10.1046/j.1365-2958.2003.03416.x. Mol Microbiol. 2003. PMID: 12622824 - Chemistry and Redox Biology of Mycothiol.
Reyes AM, Pedre B, De Armas MI, Tossounian MA, Radi R, Messens J, Trujillo M. Reyes AM, et al. Antioxid Redox Signal. 2018 Feb 20;28(6):487-504. doi: 10.1089/ars.2017.7074. Epub 2017 May 10. Antioxid Redox Signal. 2018. PMID: 28372502 Review.
Cited by
- Organic hydroperoxide resistance protein and ergothioneine compensate for loss of mycothiol in Mycobacterium smegmatis mutants.
Ta P, Buchmeier N, Newton GL, Rawat M, Fahey RC. Ta P, et al. J Bacteriol. 2011 Apr;193(8):1981-90. doi: 10.1128/JB.01402-10. Epub 2011 Feb 18. J Bacteriol. 2011. PMID: 21335456 Free PMC article. - Active and prospective latent tuberculosis are associated with different metabolomic profiles: clinical potential for the identification of rapid and non-invasive biomarkers.
Albors-Vaquer A, Rizvi A, Matzapetakis M, Lamosa P, Coelho AV, Patel AB, Mande SC, Gaddam S, Pineda-Lucena A, Banerjee S, Puchades-Carrasco L. Albors-Vaquer A, et al. Emerg Microbes Infect. 2020 Dec;9(1):1131-1139. doi: 10.1080/22221751.2020.1760734. Emerg Microbes Infect. 2020. PMID: 32486916 Free PMC article. - Characterization of myo-inositol utilization by Corynebacterium glutamicum: the stimulon, identification of transporters, and influence on L-lysine formation.
Krings E, Krumbach K, Bathe B, Kelle R, Wendisch VF, Sahm H, Eggeling L. Krings E, et al. J Bacteriol. 2006 Dec;188(23):8054-61. doi: 10.1128/JB.00935-06. Epub 2006 Sep 22. J Bacteriol. 2006. PMID: 16997948 Free PMC article. - Prioritizing genomic drug targets in pathogens: application to Mycobacterium tuberculosis.
Hasan S, Daugelat S, Rao PS, Schreiber M. Hasan S, et al. PLoS Comput Biol. 2006 Jun 9;2(6):e61. doi: 10.1371/journal.pcbi.0020061. Epub 2006 Jun 9. PLoS Comput Biol. 2006. PMID: 16789813 Free PMC article. - Steady-state and pre-steady-state kinetic analysis of Mycobacterium smegmatis cysteine ligase (MshC).
Fan F, Luxenburger A, Painter GF, Blanchard JS. Fan F, et al. Biochemistry. 2007 Oct 9;46(40):11421-9. doi: 10.1021/bi7011492. Epub 2007 Sep 12. Biochemistry. 2007. PMID: 17848100 Free PMC article.
References
- Bardarov, S., S. Bardarov, Jr., M. S. Pavelka, Jr., V. Sambandamurthy, M. Larsen, J. Tufariello, J. Chan, G. Hatfull, and W. R. Jacobs, Jr. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007-3017. - PubMed
- Buchmeier, N., A. Blanc-Potard, S. Ehrt, D. Piddington, L. Riley, and E. A. Groisman. 2000. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol. Microbiol. 35:1375-1382. - PubMed
- Buchmeier, N. A., G. L. Newton, T. Koledin, and R. C. Fahey. 2003. Association of mycothiol with protection of Mycobacterium tuberculosis from toxic oxidants and antibiotics. Mol. Microbiol. 47:1723-1732. - PubMed
- Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases