Permissive roles of phosphatidyl inositol 3-kinase and Akt in skeletal myocyte maturation - PubMed (original) (raw)
Permissive roles of phosphatidyl inositol 3-kinase and Akt in skeletal myocyte maturation
Elizabeth M Wilson et al. Mol Biol Cell. 2004 Feb.
Abstract
Skeletal muscle differentiation, maturation, and regeneration are regulated by interactions between signaling pathways activated by hormones and growth factors, and intrinsic genetic programs controlled by myogenic transcription factors, including members of the MyoD and myocyte enhancer factor 2 (MEF2) families. Insulin-like growth factors (IGFs) play key roles in muscle development in the embryo, and in the maintenance and hypertrophy of mature muscle in the adult, but the precise signaling pathways responsible for these effects remain incompletely defined. To study mechanisms of IGF action in muscle, we have developed a mouse myoblast cell line termed C2BP5 that is dependent on activation of the IGF-I receptor and the phosphatidyl inositol 3-kinase (PI3-kinase)-Akt pathway for initiation of differentiation. Here, we show that differentiation of C2BP5 myoblasts could be induced in the absence of IGF action by recombinant adenoviruses expressing MyoD or myogenin, but it was reversibly impaired by the PI3-kinase inhibitor LY294002. Similar results were observed using a dominant-negative version of Akt, a key downstream component of PI3-kinase signaling, and also were seen in C3H 10T1/2 fibroblasts. Inhibition of PI3-kinase did not prevent accumulation of muscle differentiation-specific proteins (myogenin, troponin T, or myosin heavy chain), did not block transcriptional activation of E-box containing muscle reporter genes by MyoD or myogenin, and did not inhibit the expression or function of endogenous MEF2C or MEF2D. An adenovirus encoding active Akt could partially restore terminal differentiation of MyoD-expressing and LY294002-treated myoblasts, but the resultant myofibers contained fewer nuclei and were smaller and thinner than normal, indicating that another PI3-kinase-stimulated pathway in addition to Akt is required for full myocyte maturation. Our results support the idea that an IGF-regulated PI3-kinase pathway functions downstream of or in parallel with MyoD, myogenin, and MEF2 in muscle development to govern the late steps of differentiation that lead to multinucleated myotubes.
Figures
Figure 1.
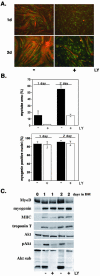
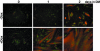
MyoD promotes differentiation of C2BP5 myoblasts. Results are shown of time course experiments using C2BP5 cells infected with Ad:MyoD or Ad:EGFP and incubated in DM for 1 or 2 d. (A) Immunocytochemistry of Ad:MyoD-infected cells for MHC (gray) and myogenin (white). Magnification, 200×. (B) Immunoblots for MyoD, myogenin, troponin T, and Akt by using whole cell protein lysates from C2BP5 cells infected with Ad:MyoD or Ad: EGFP.
Figure 2.
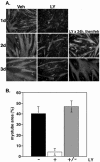
LY294002 inhibits MyoD-mediated differentiation. Results of time-course experiments are shown for C2BP5 cells infected with Ad:MyoD and incubated in DM with vehicle (dimethyl sulfoxide, DMSO) or the PI3-kinase inhibitor LY294002 in DMSO (LY, 20 μM) for 1 or 2 d. (A) Results of immunocytochemistry for MHC (red) and myogenin (green). Magnification, 200×. (B) Top, quantification of myotube area by using MHC staining (mean ± SEM of three experiments counting 20 fields at 200× magnification). Bottom, quantification of myogenin expression (mean ± SEM of 12 fields from two experiments at 200× magnification). (C) Immunoblots for MyoD, myogenin, MHC, troponin T, Akt, phospho-Akt (Ser 473) (p-Akt), and Akt substrates (Akt sub) by using whole cell protein lysates.
Figure 3.
Inhibition of differentiation by LY294002 is reversible. C2BP5 cells were infected with Ad:MyoD and incubated in DM with dimethyl sulfoxide (DMSO) (veh) or LY294002 in DMSO (LY, 20 μM) for up to 3 d as shown. (A) Results of immunocytochemistry for MHC (gray) and myogenin (dark gray). Magnification, 200×. (B) Quantification of myotube area by using MHC staining after incubation of cells for 3 d in DM with vehicle (black bars), LY (white bars), or LY for 24 h and then vehicle (+/-; gray bars). Each bar graph represents the mean ± SEM of 18 fields from three experiments at 200× magnification.
Figure 4.
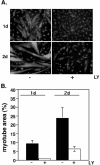
MyoD-mediated differentiation of 10T1/2 fibroblasts is impaired by LY294002. 10T1/2 fetal fibroblasts were infected with Ad:MyoD and incubated in DM with vehicle or LY294002 (LY, 20 μM) for 1 or 2 d. (A) Results of immunocytochemistry for MHC (gray). Nuclei have been stained with Hoechst dye (light gray). Magnification, 200×. (B) Quantification of myotube area by using MHC staining (mean ± SEM of 12 fields from three experiments at 200× magnification).
Figure 5.
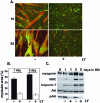
LY294002 inhibits myogenin-induced myoblast differentiation. Results are shown of C2BP5 myoblasts infected with Ad: myogenin and incubated in DM with vehicle or LY294002 (LY, 20 μM) for 1 or 2 d. (A) Immunocytochemistry of Ad:myogenin-infected cells for MHC (red) and myogenin (green). Magnification, 200×. (B) Quantification of myotube area by using MHC staining (mean ± SEM of 12 fields from three independent experiments at 200× magnification). (C) Immunoblots for myogenin, MHC, troponin T, Akt, and phospho-Akt (p-Akt) by using whole cell protein lysates from Ad:myogenin-infected C2BP5 cells.
Figure 6.
Dominant-negative Akt blocks MyoD-stimulated muscle differentiation. Results are shown of immunocytochemistry of C2BP5 myoblasts infected with Ad:MyoD and Ad:AktDN and incubated in DM for 1 or 2 d in the absence (-) or presence (+) of doxycycline (Dox). MHC staining is red and myogenin is green.
Figure 7.
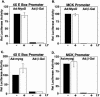
LY294002 does not block MyoD-or myogenin-stimulated gene transcription. C2BP5 cells were transfected with luciferase reporter plasmids; infected with Ad:MyoD, Ad:myogenin (Ad: myog), or Ad:β-galactosidase (Ad:β-Gal); and incubated with vehicle or LY294002 (LY, 20 μM) for 1 d, as described in MATERIALS AND METHODS. (A and C) Results with a promoter-reporter gene containing four copies of the right hand E-box element from the mouse muscle creatine kinase gene (4X E-Box). (B and D) Results with the 1.7-kb proximal promoter of the mouse muscle creatine kinase gene (MCK). Results for all panels have been normalized, with values from MyoD-expressing or myogenin-expressing cells incubated in DM being set to 100. The mean ± SEM of duplicate samples from three independent experiments is shown. For cells infected with Ad:β-Gal, the error bars are too small to be seen in the graph.
Figure 8.
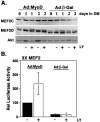
LY294002 does not alter the expression or transcriptional activity of MEF2 proteins. C2BP5 cells were infected with Ad:MyoD or Ad:β-galactosidase (Ad:β-Gal) and treated with vehicle or LY294002 (LY, 20 μM) for 1 or 2 d. (A) Results of immunoblots for MEF2C, MEF2D, and Akt by using whole cell protein lysates. (B) Results of promoter-reporter gene experiments by using a luciferase fusion gene containing three copies of the MEF2 site from the mouse muscle creatine kinase gene. Values have been normalized with results obtained from MyoD-expressing cells incubated in DM being set to 100. The mean ± SEM of duplicate samples from three independent experiments is shown.
Figure 9.
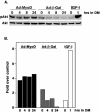
Early and sustained phosphorylation of Akt during MyoD-induced muscle differentiation. Results are shown of C2BP5 cells infected with Ad:MyoD or Ad:β-Gal and incubated in DM for up to 24 h. (A) Immunoblots are pictured for p-Akt or total Akt in a representative experiment in which noninfected myoblasts were incubated with IGF-I (2 nM concentration of the R3 analog) for 1 h. (B) The experiment shown in A has been plotted in a bar graph. Results from noninfected myoblasts incubated in DM for 1 h (small white bar) have arbitrarily been assigned a value of 1.
Figure 10.
Akt restores myocyte maturation after inhibition by LY294002. Results are shown of C2BP5 cells infected with Ad:MyoD or with both Ad:MyoD and Ad: iAkt. Cells were incubated in DM ± LY294002 (LY, 20 μM) and ± HT (1 μM) for 1 or 2 d. (A) Results of immunocytochemistry for MHC (red) and myogenin (green) after 2 d in DM. Magnification, 200×. (B) Quantification of myotube area by using MHC staining (mean ± SEM of three experiments counting a total of 12 fields at 200× magnification). (C) Immunoblots for myogenin, MHC, MEF2C, MEF2D, Akt, and phospho-Akt (Ser473) (p-Akt) by using whole cell protein lysates.
Similar articles
- Autocrine growth factor signaling by insulin-like growth factor-II mediates MyoD-stimulated myocyte maturation.
Wilson EM, Hsieh MM, Rotwein P. Wilson EM, et al. J Biol Chem. 2003 Oct 17;278(42):41109-13. doi: 10.1074/jbc.C300299200. Epub 2003 Aug 25. J Biol Chem. 2003. PMID: 12941952 - Insulin-like growth factor-mediated muscle differentiation: collaboration between phosphatidylinositol 3-kinase-Akt-signaling pathways and myogenin.
Tureckova J, Wilson EM, Cappalonga JL, Rotwein P. Tureckova J, et al. J Biol Chem. 2001 Oct 19;276(42):39264-70. doi: 10.1074/jbc.M104991200. Epub 2001 Aug 10. J Biol Chem. 2001. PMID: 11500504 - Activated raf kinase inhibits muscle cell differentiation through a MEF2-dependent mechanism.
Winter B, Arnold HH. Winter B, et al. J Cell Sci. 2000 Dec;113 Pt 23:4211-20. doi: 10.1242/jcs.113.23.4211. J Cell Sci. 2000. PMID: 11069766 - PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.
Glass DJ. Glass DJ. Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review. - Growth hormone and the insulin-like growth factor system in myogenesis.
Florini JR, Ewton DZ, Coolican SA. Florini JR, et al. Endocr Rev. 1996 Oct;17(5):481-517. doi: 10.1210/edrv-17-5-481. Endocr Rev. 1996. PMID: 8897022 Review.
Cited by
- Muscle development, regeneration and laminopathies: how lamins or lamina-associated proteins can contribute to muscle development, regeneration and disease.
Dubinska-Magiera M, Zaremba-Czogalla M, Rzepecki R. Dubinska-Magiera M, et al. Cell Mol Life Sci. 2013 Aug;70(15):2713-41. doi: 10.1007/s00018-012-1190-3. Epub 2012 Nov 10. Cell Mol Life Sci. 2013. PMID: 23138638 Free PMC article. Review. - Characterizing a distal muscle enhancer in the mouse Igf2 locus.
Alzhanov D, Rotwein P. Alzhanov D, et al. Physiol Genomics. 2016 Feb;48(2):167-72. doi: 10.1152/physiolgenomics.00095.2015. Epub 2015 Dec 8. Physiol Genomics. 2016. PMID: 26645089 Free PMC article. - Differential regulation of IGF-I and IGF-II gene expression in skeletal muscle cells.
Jiao S, Ren H, Li Y, Zhou J, Duan C, Lu L. Jiao S, et al. Mol Cell Biochem. 2013 Jan;373(1-2):107-13. doi: 10.1007/s11010-012-1479-4. Epub 2012 Oct 10. Mol Cell Biochem. 2013. PMID: 23054195 - NET37, a nuclear envelope transmembrane protein with glycosidase homology, is involved in myoblast differentiation.
Datta K, Guan T, Gerace L. Datta K, et al. J Biol Chem. 2009 Oct 23;284(43):29666-76. doi: 10.1074/jbc.M109.034041. Epub 2009 Aug 25. J Biol Chem. 2009. PMID: 19706595 Free PMC article. - Clearance of defective muscle stem cells by senolytics restores myogenesis in myotonic dystrophy type 1.
Conte TC, Duran-Bishop G, Orfi Z, Mokhtari I, Deprez A, Côté I, Molina T, Kim TY, Tellier L, Roussel MP, Maggiorani D, Benabdallah B, Leclerc S, Feulner L, Pellerito O, Mathieu J, Andelfinger G, Gagnon C, Beauséjour C, McGraw S, Duchesne E, Dumont NA. Conte TC, et al. Nat Commun. 2023 Jul 19;14(1):4033. doi: 10.1038/s41467-023-39663-3. Nat Commun. 2023. PMID: 37468473 Free PMC article.
References
- Apone, S., and Hauschka, S.D. (1995). Muscle gene E-box control elements. Evidence for quantitatively different transcriptional activities and the binding of distinct regulatory factors. J. Biol. Chem. 270, 21420-21427. - PubMed
- Bennett, A.M., and Tonks, N.K. (1997). Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278, 1288-1291. - PubMed
- Black, B.L., and Olson, E.N. (1998). Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell. Dev. Biol. 14, 167-196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases