Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons - PubMed (original) (raw)
Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons
Hussam Jourdi et al. Dev Biol. 2003.
Abstract
Postsynaptic molecules with PDZ domains (PDZ proteins) interact with various glutamate receptors and regulate their subcellular trafficking and stability. In rat neocortical development, the protein expression of AMPA-type glutamate receptor GluR1 lagged behind its mRNA expression and rather paralleled an increase in PDZ protein levels. One of the neurotrophins, brain-derived neurotrophic factor (BDNF), appeared to contribute to this process, regulating the PDZ protein expression. In neocortical cultures, BDNF treatment upregulated SAP97, GRIP1, and Pick1 PDZ proteins. Conversely, BDNF gene targeting downregulated these same PDZ molecules. The BDNF-triggered increases in PDZ proteins resulted in the elevation of their total association with the AMPA receptors GluR1 and GluR2/3, which led to the increase in AMPA receptor proteins. When Sindbis viruses carrying GluR1 or GluR2 C-terminal decoys disrupted their interactions, GluR2 C-terminal decoys inhibited both BDNF-triggered GluR1 and GluR2/3 increases, whereas GluR1 C-terminal decoys blocked only the BDNF-triggered GluR1 increase. In agreement, coexpression of SAP97 and GluR1 in nonneuronal HEK293 cells increased both proteins compared with their single transfection, implying mutual stabilization. This work reveals a novel function of BDNF in postsynaptic development by regulating the PDZ protein expression.
Figures
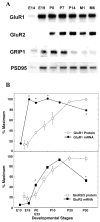
Fig. 1
Developmental regulation of AMPA receptor subunits and PDZ proteins. Relative protein levels for GluR1, GluR2/3, GRIP1, SAP97, and PSD95 (panPDZ) were determined in rat neocortex by immunoblotting. Rat cerebral cortex was isolated from rats at various ages and total protein was extracted. Protein (40 _μ_g) was separated on a 7.5% SDS-PAGE and blotted onto a nitrocellulose membrane. Lanes correspond to embryonic days (E16 and E18) and postnatal days (P0, P5, P10, P20, P30, and P60).
Fig. 2
mRNA expression of AMPA receptors and PDZ proteins during rat neocortical development. (A) Brain mRNA levels for GluR1, GluR2, GRIP1, and PSD95 were examined during rat development by RNA blotting. Rat cerebral cortex was isolated from rats at various ages, and total RNA was extracted. PolyA + RNA (1.8 _μ_g) was separated on a 1.5% formamide agarose gel and blotted onto a nylon membrane. Lanes correspond to embryonic days (E14 and E19), postnatal days (P0, P7, and P14), and postnatal months (M1 and M6) from left to right. (B) mRNA levels for GluR1 and GluR2 were determined by densitometric analysis of the blots and compared with their protein levels. The protein levels were similarly measured by three independent immunoblots as shown in Fig. 1. *P < 0.05, out of ±2 SD range.
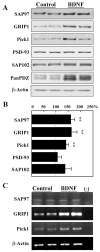
Fig. 3
BDNF differentially regulates the expression of various PDZ proteins. Neocortical neurons were cultured in serum-free medium in the presence or absence of BDNF (50 ng/ml) for 5 days. Total cellular protein was collected and processed for immunoblotting. (A) Typical examples of immunoblotting are displayed. (B) The blotting results of similar culture experiments (four cultures and blots with duplicated lanes; total n = 8) were quantified by densitometry and plotted. (C) cDNA bands amplified by RT-PCR for the indicated molecules showed that BDNF chronic application resulted in increased signals for GRIP1 and Pick1. The same treatment did not modify SAP97 nor _β_-Actin mRNAs. “−” represents PCR reaction without mRNA as a negative control. mRNA was derived from two independent cultures. **P < 0.005 with the Mann-Whitney _U_-test.
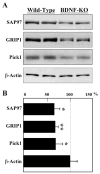
Fig. 4
Impaired expression of PDZ proteins in the neocortex of BDNF-gene knockout mice. On postnatal day 1 the neocortex was dissected from homozygous BDNF knockout mice and from wild-type littermates. SAP97, GRIP1, and Pick1 protein levels were analyzed by immunoblotting. (A) The expression of _β_-actin was not affected by the lack of BDNF. (B) Total levels of PDZ proteins were quantified by densitometry and compared in the wild type and BDNF-homozygous mutant mice (n = 6 mice, each). *P < 0.05; **P < 0.005 with the Mann-Whitney _U_-test.
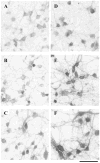
Fig. 5
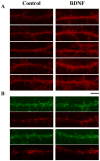
BDNF enhanced PDZ protein immunoreactivity in cultured neocortical neurons. Control (A–C) or BDNF-treated (D–F) neocortical neurons in culture were fixed and incubated with anti-SAP97 antibody (A, D), anti-GRIP1 antibody (B, E), or anti-PSD95/panPDZ antibody (C, F). Immunoreactivity was visualized with the DAB-peroxidase technique. Chronic incubation with BDNF (50 ng/ml daily for 5 days) significantly enhanced the staining with the three antibodies. Cell density was 3500 ± 400 cells/mm2 in control culture and 3100 ± 300 cells/mm2 in BDNF-treated culture. Scale bar, 30 _μ_m.
Fig. 6
Effects of BDNF on the immunoreactivity for PDZ proteins in mature cortical culture. Lower density neocortical cultures were grown for 2 weeks with or without BDNF. After fixation, immunoreactivity for the anti-PSD95/panPDZ antibody was visualized with Alexa 546-conjugated secondary antibody (red) (A). (B) Immunostaining of presynaptic structures was marked with the mixture of anti-synaptobrevin and anti-synaptophysin monoclonal antibodies followed by the Alexa 546-conjugated secondary antibody (red). Immunostaining for SAP97 was simultaneously visualized with the Alexa 488-conjugated secondary antibody (green). Dendritic processes were randomly pictured (n = 15 each for A and B) and immunoreactive spots along dendritic processes were counted. Densities of SAP97-positive spots and synaptophysin/synaptobrevin-positive terminals were 243.4 ± 71.0 and 295.4 ± 102.8 spots/mm dendrite in the BDNF-treated culture and 289.2 ± 114.8 and 245.2 ± 118.6 spots/mm dendrite in control, respectively; P = 0.67 for SAP97-positive spots and 0.83 for synaptophysin/synaptobrevin-positive spots. Pictures of dendrites having higher immunoreactivities were chosen for display (A; n = 5 each, B; n = 2 each). Scale bar, 10 _μ_m.
Fig. 7
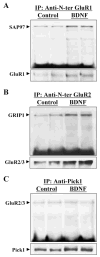
BDNF enhanced the association of GluR1 and GluR2/3 with SAP97 and GRIP1 in neocortical neuronal cultures. Neurons were incubated, with or without BDNF (50 ng/ml), for 5 days. Protein samples were 1% DOC-extracted and used for coimmunoprecipitation (co-IP). Co-IP of SAP97 with anti-N-terminal GluR1 antibody (A), GRIP1 with anti-N-terminal GluR2 antibody (B), or GluR2/3 with anti-Pick1 antibody (C) revealed that only SAP97-GluR1 and GRIP1-GluR2/3 interactions were enhanced by BDNF in the treated cultures. To control the above co-IP reaction, immunoblots were reprobed with anti C-terminal GluR1 and anti C-terminal GluR2/3 antibodies (lower panels in A and B). Because of the similarity in the molecular sizes of Pick1 and the immunoglobulin heavy chain, the co-IP reaction was started with the anti-Pick1 antibody and immunoblotting was carried out with anti-GluR2/3. In addition, total cellular lysates were immunoblotted with anti-Pick1 antibody, which revealed that Pick1 protein was enhanced by BDNF (C). Each lane represents independent sister culture.
Fig. 8
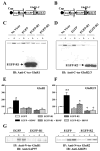
Overexpression of GluR1 and GluR2 C-terminal decoys disrupted the BDNF-mediated increase in AMPA receptors. Neocortical neurons were cultured for 4 days and then incubated with (+) or without (−) BDNF for the last 24 h before harvesting. The shorter BDNF treatment was still able to mimic a similar magnitude of the protein increase in AMPA receptors. During the incubation with BDNF, neurons were infected with the Sindbis viruses coding for EGFP alone, EGFP-R1 (A), or for EGFP-R2 (B) (Iwakura et al., 2001) to study the consequences of disrupting AMPA receptors’ interaction with their associated PDZ proteins. At the viral titre used (> 1 × 107), more than 90% of the cells were infected, as monitored by EGFP fluorescence (Iwakura et al., 2001). The control EGFP construct was essentially the same as the EGFP-R1 and EGFP-R2 constructs but lacked the decoy peptides. (A and B) nsp, nonstructural proteins; psg, subgenomic promoter; and poly A; poly-adenylated transcript tail. Hatched boxes fused to the EGFP represent the C-terminal decoys of GluR1 and GluR2 (short splice variant). (C) Probing with anti-C-terminal GluR1 revealed the native GluR1 band as well as a lower band corresponding to the expected size of EGFP-R1 fusion protein (28 kDa). (D) Similarly, the BDNF-induced increase in GluR2/3 protein was challenged by the overexpression of EGFP-R2 fusion protein. Statistical analysis of the GluR1 (E) and GluR2/3 (F) immunoreactivities between pairs of BDNF-plus and BDNF-minus cultures (n = 3). GluR1 or GluR2/3 immunoreactivity in virus-free control culture was set as 100%. Student’s _t_-test was applied to comparison between BDNF-plus and BDNF-minus cultures (*P < 0.05; **P < 0.01) or between the cultures transfected with EGFP-R2 and EGFP alone (+P < 0.05). (G) The overexpression of EGFP-R1 and EGFP-R2 decoys reduced the BDNF-stimulated interaction between GluR1 and SAP97 and between GluR2/3 and GRIP1, respectively, compared with that in the EGFP overexpression. Cell lysates were prepared from infected neocortical cultures and treated with anti-N-terminal GluR1 antibody or anti-N-terminal GluR2 antibody to immunoprecipiate the complexes of AMPA receptors and PDZ protein(s).
Fig. 9
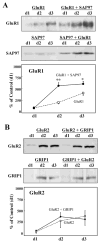
Effects of coexpression of SAP97 and GRIP1 proteins on AMPA receptor levels in HEK293 cells. cDNA for GluR1 or GluR2 in expression vector plasmids carrying the CMV promoter was transfected into human embryonic kidney cells, HEK293, together with that for SAP97 or GRIP1, respectively. Their transient expressions were monitored on days 1, 2, and 3 by immunoblotting. cDNAs used in each experiment are indicated on top of each blot. Three independent experiments were performed, and protein levels for GluR1 or GluR2 were quantified by densitometry for statistical analysis. Significant interaction between GluR1 and SAP97, or GluR2 and GRIP1, was indeed detected in the immunoprecipiates with the anti-N-terminal GluR1 antibody or the anti-N-terminal GluR2 antibody, respectively (data not shown). Results were obtained from three sets of cultures (n = 3). *P < 0.05; **P < 0.005 with paired Student’s _t_-test.
Similar articles
- Acute BDNF treatment upregulates GluR1-SAP97 and GluR2-GRIP1 interactions: implications for sustained AMPA receptor expression.
Jourdi H, Kabbaj M. Jourdi H, et al. PLoS One. 2013;8(2):e57124. doi: 10.1371/journal.pone.0057124. Epub 2013 Feb 27. PLoS One. 2013. PMID: 23460828 Free PMC article. - SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit.
Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. Leonard AS, et al. J Biol Chem. 1998 Jul 31;273(31):19518-24. doi: 10.1074/jbc.273.31.19518. J Biol Chem. 1998. PMID: 9677374 - BDNF-induced local protein synthesis and synaptic plasticity.
Leal G, Comprido D, Duarte CB. Leal G, et al. Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review. - The functions of repressor element 1-silencing transcription factor in models of epileptogenesis and post-ischemia.
Butler-Ryan R, Wood IC. Butler-Ryan R, et al. Metab Brain Dis. 2021 Aug;36(6):1135-1150. doi: 10.1007/s11011-021-00719-2. Epub 2021 Apr 4. Metab Brain Dis. 2021. PMID: 33813634 Free PMC article. Review.
Cited by
- Long-term Administration of Salicylate-induced Changes in BDNF Expression and CREB Phosphorylation in the Auditory Cortex of Rats.
Yi B, Wu C, Shi R, Han K, Sheng H, Li B, Mei L, Wang X, Huang Z, Wu H. Yi B, et al. Otol Neurotol. 2018 Mar;39(3):e173-e180. doi: 10.1097/MAO.0000000000001717. Otol Neurotol. 2018. PMID: 29342042 Free PMC article. - The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors.
Palmer CL, Cotton L, Henley JM. Palmer CL, et al. Pharmacol Rev. 2005 Jun;57(2):253-77. doi: 10.1124/pr.57.2.7. Pharmacol Rev. 2005. PMID: 15914469 Free PMC article. Review. - Brain-derived neurotrophic factor activation of CaM-kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1-containing calcium-permeable AMPA receptors.
Fortin DA, Srivastava T, Dwarakanath D, Pierre P, Nygaard S, Derkach VA, Soderling TR. Fortin DA, et al. J Neurosci. 2012 Jun 13;32(24):8127-37. doi: 10.1523/JNEUROSCI.6034-11.2012. J Neurosci. 2012. PMID: 22699894 Free PMC article. - Postsynaptic TrkB signaling has distinct roles in spine maintenance in adult visual cortex and hippocampus.
Chakravarthy S, Saiepour MH, Bence M, Perry S, Hartman R, Couey JJ, Mansvelder HD, Levelt CN. Chakravarthy S, et al. Proc Natl Acad Sci U S A. 2006 Jan 24;103(4):1071-6. doi: 10.1073/pnas.0506305103. Epub 2006 Jan 17. Proc Natl Acad Sci U S A. 2006. PMID: 16418274 Free PMC article. - Promotion of structural plasticity in area V2 of visual cortex prevents against object recognition memory deficits in aging and Alzheimer's disease rodents.
Navarro-Lobato I, Masmudi-Martín M, López-Aranda MF, López-Téllez JF, Delgado G, Granados-Durán P, Gaona-Romero C, Carretero-Rey M, Posadas S, Quiros-Ortega ME, Khan ZU. Navarro-Lobato I, et al. Neural Regen Res. 2024 Aug 1;19(8):1835-1841. doi: 10.4103/1673-5374.389301. Epub 2023 Nov 8. Neural Regen Res. 2024. PMID: 38103251 Free PMC article.
References
- Aoki C, Wu K, Elste A, Len Gw, Lin Sy, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59:454–463. - PubMed
- Arai Y, Mizuguchi M, Takashima S. Developmental changes of glutamate receptors in the rat cerebral cortex and hippocampus. Anat Embryol (Berl) 1997;195:65–70. - PubMed
- Boulter J, Hollmann M, O’Shea-Greenfield A, Hartley M, Deneris E, Maron C, Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990;249:1033–1037. - PubMed
- Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. - PubMed
- Causing CG, Gloster A, Aloyz R, Banji SX, Chang E, Fawcett J, Kuchel G, Miller FD. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases