Regulation of the chemokine receptor CXCR4 by hypoxia - PubMed (original) (raw)
. 2003 Nov 3;198(9):1391-402.
doi: 10.1084/jem.20030267.
Badarch Uranchimeg, Alessandra Saccani, Subhra K Biswas, Andrea Doni, Annamaria Rapisarda, Sergio Bernasconi, Simona Saccani, Manuela Nebuloni, Luca Vago, Alberto Mantovani, Giovanni Melillo, Antonio Sica
Affiliations
- PMID: 14597738
- PMCID: PMC2194248
- DOI: 10.1084/jem.20030267
Regulation of the chemokine receptor CXCR4 by hypoxia
Tiziana Schioppa et al. J Exp Med. 2003.
Abstract
Cell adaptation to hypoxia (Hyp) requires activation of transcriptional programs that coordinate expression of genes involved in oxygen delivery (via angiogenesis) and metabolic adaptation (via glycolysis). Here, we describe that oxygen availability is a determinant parameter in the setting of chemotactic responsiveness to stromal-derived factor 1 (CXCL12). Low oxygen concentration induces high expression of the CXCL12 receptor, CXC receptor 4 (CXCR4), in different cell types (monocytes, monocyte-derived macrophages, tumor-associated macrophages, endothelial cells, and cancer cells), which is paralleled by increased chemotactic responsiveness to its specific ligand. CXCR4 induction by Hyp is dependent on both activation of the Hyp-inducible factor 1 alpha and transcript stabilization. In a relay multistep navigation process, the Hyp-Hyp-inducible factor 1 alpha-CXCR4 pathway may regulate trafficking in and out of hypoxic tissue microenvironments.
Figures
Figure 1.
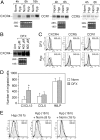
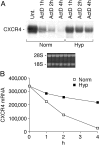
Effect of hypoxia (Hyp) on CXCR4 expression by fresh human monocytes. (A) Fresh human monocytes obtained from peripheral blood of healthy donors were cultured for different times in normoxia (Norm) or hypoxic (Hyp) conditions, as indicated. Total RNA was analyzed by Northern blot for CXCR4, CCR1, and CCR5 mRNA expression. (B) Total RNA from fresh human monocytes cultured for 4 h in the presence of increasing concentration of DFX was analyzed by Northern blot. (C) Hyp- and DFX-induced CXCR4 surface expression in fresh human monocytes. Cells were cultured for 16 h in the indicated conditions and analyzed for CXCR4 surface expression. Surface expression was determined by flow cytometry using a mouse monoclonal antibody anti–human CXCR4. The results are representative of three independent experiments. (dotted line) Irrelevant antibody. (continuous line) Norm. (shaded region) Hyp or DFX. (D) Effect of DFX on the chemotactic response of monocytes to 100 ng/ml CXCL12, 100 ng/ml CCL5, and 10−8 M FMLP. Cells were cultured for 16 h in the presence of 400 μM DFX. Migration of monocytes was assayed by chemotaxis microchamber technique. Results are mean ± SD of five experiments. *, P < 0.05 versus cells cultured in normoxic conditions (paired Student's t test). (E) Effect of reoxygenation on CXCR4 surface expression. Fresh human monocytes were cultured for 16 h in Hyp and subsequently reexposed to Norm for the indicated times. CXCR4 surface expression was determined by flow cytometry.
Figure 2.
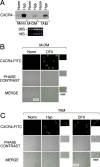
Effect of Hyp on CXCR4 expression by fresh human monocytes (mono), monocyte-derived macrophages (MDMs), and tumor-associated macrophages (TAMs). (A) Cells were cultured for 4 h in Hyp, and total RNA was analyzed by Northern blot for CXCR4 mRNA expression. MDM (B) and TAMs from ascitic fluid of human ovarian cancer (C) were cultured for 16 h in Norm, Hyp, or in the presence of 400 μM DFX, as indicated. CXCR4 surface staining was performed and detected by laser confocal microscopy. Staining with both isotype-matched control antibodies was done for all samples (not depicted).
Figure 3.
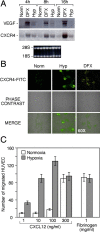
Effect of Hyp on CXCR4 expression by human endothelial venules (HUVECs). (A) Cells were cultured for 4, 8, and 16 h under Norm, Hyp, or in the presence of 400 μM DFX, respectively. Thereafter, total RNA was analyzed by Northern blot for CXCR4 and VEGF mRNAs expression. (B) HUVECs were cultured for 16 h in Norm, Hyp, and in the presence of 400 μM DFX, as indicated. CXCR4 surface staining was performed and detected by laser confocal microscopy. (C) Effect of Hyp on the chemotactic response of HUVECs to CXCL12. Cells were cultured for 16 h in hypoxic conditions. Fibrinogen was used as a reference attractant. Results are mean ± SD of three experiments.
Figure 4.
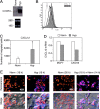
Effects of Hyp on CXCR4 expression by cancer cells. The ovarian cancer cell line CAOV3 was cultured in normoxic or hypoxic conditions and analyzed respectively for both CXCR4 mRNA expression (A) and CXCR4 surface expression (B). (A) cells were cultured for 4 h in normoxia (Norm) or hypoxic (Hyp) conditions, as indicated. Total RNA was analyzed by Northern blot for CXCR4. (B) Cells were cultured for 16 h in Norm or hypoxic (Hyp) conditions. After this period, CXCR4 surface expression was determined by flow cytometry. (dotted line) Irrelevant antibody. (continuous line) Norm. (shaded region) Hyp. (C) Effect of Hyp on the chemotactic response of CAOV3 ovarian cancer cells to 100 ng/ml CXCL12. Cells were incubated overnight in Hyp condition and migration determined by Transwells, as described in Materials and Methods. Results are mean ± SD of three experiments. *, P < 0.05 versus cells cultured in normoxic conditions (Paired Student's t test). (D) Effects of Hyp on CXCL12 mRNA expression by MCF-7 and CAOV3 cells. Cells were incubated for 4 h in Norm and Hyp as indicated, and CXCR4 gene expression was next determined by real-time PCR. Results are representative of two independent experiments. Values are expressed as fold increases relative to the reference sample (Norm). (E) Sustained Hyp-induced CXCR4 expression upon reoxygenation. CAOV3 cells were cultured under Norm or Hyp conditions for 16 h. Thereafter, the cells were exposed to Norm for a further 14 h and stained for CXCR4 expression. The figure shows a representative field using confocal microscopy. CAOV3 cells stained for CXCR4 (blue fluorescence) and nuclei (red fluorescence). The bottom panels in each group shows phase-contrast images merged with fluorescence readings.
Figure 5.
Role of HIF-1α in the regulation of CXCR4 gene expression. (A) Expression of CXCR4 in HIF-1α KO mouse embryo fibroblast. Mouse embryo fibroblast from wild type (MEF+/+) or knockout for the α subunit of HIF-1 (MEF−/−) were incubated under normoxic or hypoxic conditions for 6 h, and total RNA was tested for VEGF and CXCR4 mRNA levels by real-time PCR. (B) DFX was used as Hyp-inducing agent. Results are the average of three independent experiments. (C) Expression of CXCR4 in VHL WT and mutated renal carcinoma cells. Expression of CXCR4 and VEGF mRNAs was tested by real-time PCR in the renal cancer cell line 786.0 (VHL mutated) and WT2 (in which a WT VHL has been reintroduced). Results are the average of three independent experiments. (D) HIF-1–dependent transcriptional activation of CXCR4 promoter. MCF-7 breast carcinoma cells were transiently transfected with a plasmid containing a 2.6 kb fragment of the CXCR4 promoter linked to the luciferase reporter gene, with or without a HIF-1α expression vector. Cells were incubated under normoxic or hypoxic conditions for 24 h and evaluated for the luciferase activity. Results are the average of three independent experiments. (E) Hyp-induced HIF-1α recruitment to the CXCR4 promoter. CAOV3 cells transfected with the p(HA)HIF-1α plasmid were cultured for 4 h in normoxic or hypoxic conditions. ChIP was performed to investigate the recruitment of HIF-1α on the CXCR4 promoter. (lane 1) Untransfected. (lane 2) Norm. (lane 3) Hyp.
Figure 6.
Stabilization of CXCR4 mRNA by Hyp. (A) Fresh human monocytes were cultured for 4 h under Norm or Hyp, and in the presence or absence of 1 μg/ml actinomycin D (ActD). Thereafter, total RNA was extracted at different times as indicated and analyzed by Northern blot for CXCR4 mRNA expression. (B) Densitometric analysis: CXCR4 mRNA levels are expressed as arbitrary units. In this experiment, the basal CXCR4 mRNA level appears higher compared with other experiments, as we exposed the film for a longer period for better visualization of the blot.
Similar articles
- Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis.
Pan J, Mestas J, Burdick MD, Phillips RJ, Thomas GV, Reckamp K, Belperio JA, Strieter RM. Pan J, et al. Mol Cancer. 2006 Nov 3;5:56. doi: 10.1186/1476-4598-5-56. Mol Cancer. 2006. PMID: 17083723 Free PMC article. - Expression and regulation of stromal cell-derived factor-1 (SDF1) and chemokine CXC motif receptor 4 (CXCR4) in equine and bovine preovulatory follicles.
Sayasith K, Sirois J. Sayasith K, et al. Mol Cell Endocrinol. 2014 Jun 25;391(1-2):10-21. doi: 10.1016/j.mce.2014.04.009. Epub 2014 Apr 28. Mol Cell Endocrinol. 2014. PMID: 24784705 - HGF induces CXCR4 and CXCL12-mediated tumor invasion through Ets1 and NF-kappaB.
Maroni P, Bendinelli P, Matteucci E, Desiderio MA. Maroni P, et al. Carcinogenesis. 2007 Feb;28(2):267-79. doi: 10.1093/carcin/bgl129. Epub 2006 Jul 13. Carcinogenesis. 2007. PMID: 16840440 - Chemokine receptor CXCR4: role in gastrointestinal cancer.
Lombardi L, Tavano F, Morelli F, Latiano TP, Di Sebastiano P, Maiello E. Lombardi L, et al. Crit Rev Oncol Hematol. 2013 Dec;88(3):696-705. doi: 10.1016/j.critrevonc.2013.08.005. Epub 2013 Aug 28. Crit Rev Oncol Hematol. 2013. PMID: 24120239 Review. - Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration.
Bosco MC, Puppo M, Blengio F, Fraone T, Cappello P, Giovarelli M, Varesio L. Bosco MC, et al. Immunobiology. 2008;213(9-10):733-49. doi: 10.1016/j.imbio.2008.07.031. Epub 2008 Sep 21. Immunobiology. 2008. PMID: 18926289 Review.
Cited by
- CXCR4 Regulates Extra-Medullary Myeloma through Epithelial-Mesenchymal-Transition-like Transcriptional Activation.
Roccaro AM, Mishima Y, Sacco A, Moschetta M, Tai YT, Shi J, Zhang Y, Reagan MR, Huynh D, Kawano Y, Sahin I, Chiarini M, Manier S, Cea M, Aljawai Y, Glavey S, Morgan E, Pan C, Michor F, Cardarelli P, Kuhne M, Ghobrial IM. Roccaro AM, et al. Cell Rep. 2015 Jul 28;12(4):622-35. doi: 10.1016/j.celrep.2015.06.059. Epub 2015 Jul 16. Cell Rep. 2015. PMID: 26190113 Free PMC article. - Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis.
Cheng NC, Chen SY, Li JR, Young TH. Cheng NC, et al. Stem Cells Transl Med. 2013 Aug;2(8):584-94. doi: 10.5966/sctm.2013-0007. Epub 2013 Jul 11. Stem Cells Transl Med. 2013. PMID: 23847001 Free PMC article. - RNA interference-mediated silencing of speckle-type POZ protein promotes apoptosis of renal cell cancer cells.
Liu X, Sun G, Sun X. Liu X, et al. Onco Targets Ther. 2016 Apr 20;9:2393-402. doi: 10.2147/OTT.S91097. eCollection 2016. Onco Targets Ther. 2016. PMID: 27143934 Free PMC article. - The impact of the stromal cell-derived factor-1-3'A and E-selectin S128R polymorphisms on breast cancer.
Kontogianni P, Zambirinis CP, Theodoropoulos G, Gazouli M, Michalopoulos NV, Flessas J, Liberi M, Zografos GC. Kontogianni P, et al. Mol Biol Rep. 2013 Jan;40(1):43-50. doi: 10.1007/s11033-012-1989-x. Epub 2012 Nov 6. Mol Biol Rep. 2013. PMID: 23129313 - The CXCR4-CXCL12 axis in Ewing sarcoma: promotion of tumor growth rather than metastatic disease.
Berghuis D, Schilham MW, Santos SJ, Savola S, Knowles HJ, Dirksen U, Schaefer KL, Vakkila J, Hogendoorn PC, Lankester AC. Berghuis D, et al. Clin Sarcoma Res. 2012 Dec 18;2(1):24. doi: 10.1186/2045-3329-2-24. Clin Sarcoma Res. 2012. PMID: 23249693 Free PMC article.
References
- Semenza, G.L. 2001. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 7:345–350. - PubMed
- Semenza, G.L. 1999. Perspectives on oxygen sensing. Cell. 98:281–284. - PubMed
- Grimshaw, M.J., and F.R. Balkwill. 2001. Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation–a potential mechanism. Eur. J. Immunol. 31:480–489. - PubMed
- Turner, L., C. Scotton, R. Negus, and F. Balkwill. 1999. Hypoxia inhibits macrophage migration. Eur. J. Immunol. 29:2280–2287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources