Electroporation and RNA interference in the rodent retina in vivo and in vitro - PubMed (original) (raw)
Electroporation and RNA interference in the rodent retina in vivo and in vitro
Takahiko Matsuda et al. Proc Natl Acad Sci U S A. 2004.
Abstract
The large number of candidate genes made available by comprehensive genome analysis requires that relatively rapid techniques for the study of function be developed. Here, we report a rapid and convenient electroporation method for both gain- and loss-of-function studies in vivo and in vitro in the rodent retina. Plasmid DNA directly injected into the subretinal space of neonatal rodent pups was taken up by a significant fraction of exposed cells after several pulses of high voltage. With this technique, GFP expression vectors were efficiently transfected into retinal cells with little damage to the operated pups. Transfected GFP allowed clear visualization of cell morphologies, and the expression persisted for at least 50 days. DNA-based RNA interference vectors directed against two transcription factors important in photoreceptor development led to photoreceptor phenotypes similar to those of the corresponding knockout mice. Reporter constructs carrying retinal cell type-specific promoters were readily introduced into the retina in vivo, where they exhibited the appropriate expression patterns. Plasmid DNA was also efficiently transfected into retinal explants in vitro by high-voltage pulses.
Figures
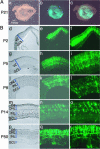
Fig. 1.
In vivo electroporated rat retinae harvested at various developmental stages. (A) Whole-mount preparation of rat retina in vivo electroporated with pCAG-GFP at P0 and harvested at P21. Pictures were taken from the scleral side. (B) Rat retinae in vivo electroporated with pCAG-GFP at P0 were harvested at P2 (d_–_f), P5 (g_–_i), P8 (j_–_l), P14 (m_–_o), or P50 (p_–_r), and cryosections were prepared.
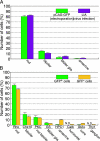
Fig. 2.
Cell type composition of retinal cells labeled by in vivo electroporation. (A) Cell type composition determined based on morphologies and locations in the retina. Rat retinae electroporated in vivo with pCAG-GFP, or infected in vivo with the replication-incompetent LIA retrovirus at P0, were harvested at P14 and sectioned. The LIA-infected retinae expressing alkaline phosphatase (AP) were stained histochemically for AP activity. Green bars represent the retinal cells electroporated with the pCAG-GFP plasmid. Purple bars represent the retinal cells infected with the LIA retrovirus. (B) Cell type composition determined by immunostaining. Rat retinae electroporated in vivo with pCAG-GFP at P0 were harvested and dissociated into single cells at P14. Dissociated cells were stained with anti-rhodopsin (rod PR), anti-Chx10 (bipolar), anti-protein kinase Cα (rod bipolar), anti-glutamine synthetase (Müller glia), anti-HPC-1 (amacrine), anti-calbindin (horizontal), anti-Gt2α (cone PR), or anti Thy-1 (GC), and the numbers of positive cells were scored. Both GFP-positive (green bars) and GFP-negative (yellow bars) cells were analyzed.
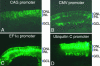
Fig. 3.
Comparison of ubiquitous promoters in the developing rat retina. Rat retinae were electroporated in vivo at P0 with the GFP expression vectors driven by CAG promoter (A), CMV promoter (B), human EF1α promoter (C), or human ubiquitin C promoter (D) and sectioned at P10.
Fig. 4.
In vitro electroporated mouse retinal explants. (A) Mouse retinae of P0 CD1 (a and b), adult CD1 (c and d), or adult Swiss–Webster having a retinal degeneration mutation (e and f) were in vitro electroporated with pCAG-GFP from the scleral side (a, c, and e) or from the vitreal side (b, d, and f) and cultured for 5 days. Similar results were observed 16 h after electroporation. (B) A section of CD1 mouse retina in vitro electroporated with pCAG-GFP at P0 and cultured for 10 days.
Fig. 5.
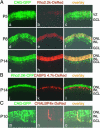
Cell type-specific labeling using rhodopsin, CABP5, and CRALBP promoters. (A) Rat retinae were coelectroporated in vivo with pCAG-GFP (3μg/μl) and bovine rhodopsin promoter 2.2K-DsRed (pRho-2.2K-DsRed, 3 μg/μl) at P0, harvested at P5 (a_–_c), P8 (d_–_f), or P14 (g_–_i), and sectioned. (B) Rat retinae were coelectroporated in vivo with pRho-2.2K-CFP (3 μg/μl) and mouse CABP5 promoter 4.7K-DsRed (pCABP5-4.7K-DsRed, 3 μg/μl) at P0, harvested at P14, and sectioned. (C) Rat retinae were coelectroporated in vivo with pCAG-GFP (3 μg/μl) and mouse CRALBP promoter 4.0K-DsRed (pCRALBP-4.0K-DsRed, 3 μg/μl) at P0, harvested at P10, and sectioned. Some DsRed-positive Müller glial cells were truncated in the process of cutting sections.
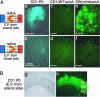
Fig. 6.
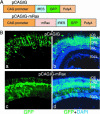
Ectopic expression of Rax using the IRES-GFP vector. (A) Structures of pCAGIG and pCAGIG-mRax vectors. (B) Rat retinae in vivo electroporated with pCAGIG (a and b) or pCAGIG-mRax (c and d) at P0 were harvested at P21 and sectioned. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Fig. 7.
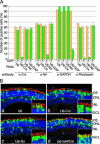
Knockdown of Crx and Nrl expressions in the mouse retina using RNAi vectors. (A) Specific down-regulation of the expression of Crx, Nrl, GAPDH, and rhodopsin in the RNAi vector transfected mouse retinae. CD1 mouse retinae were coelectroporated in vivo with pCAG-GFP (2 μg/μl) and an RNAi vector (U6, U6-Crx, U6-Nrl or U6-GAPDH; 4 μg/μl) at P0, harvested at P10, and dissociated into single cells. The dissociated cells were stained with anti-Crx, anti-Nrl, anti-GAPDH, or anti-rhodopsin antibody, and the numbers of positive cells were scored. Both GFP-positive (green bars) and GFP-negative (yellow bars) cells were analyzed. (B) Morphology of the retinal cells transfected with RNAi vector. CD1 mouse retinae were coelectroporated in vivo with pCAG-GFP and an RNAi vector at P0, harvested at P20, and sectioned. Sections were stained with anti-rhodopsin antibody (red) and 4′,6-diamidino-2-phenylindole (blue), and images were taken with a confocal microscope (Zeiss LSM 510). (Insets) Higher-magnification views of OSs. (Magnifications: ×800.)
Similar articles
- [In vivo electroporation of newborn mouse retina].
Lirong X, Danian C, Naihong Y. Lirong X, et al. Yi Chuan. 2014 Nov;36(11):1173-8. doi: 10.3724/SP.J.1005.2014.1173. Yi Chuan. 2014. PMID: 25567876 Chinese. - Analysis of gene function in the retina.
Matsuda T, Cepko CL. Matsuda T, et al. Methods Mol Biol. 2008;423:259-78. doi: 10.1007/978-1-59745-194-9_19. Methods Mol Biol. 2008. PMID: 18370205 - Ex vivo electroporation of retinal cells: a novel, high efficiency method for functional studies in primary retinal cultures.
Vergara MN, Gutierrez C, O'Brien DR, Canto-Soler MV. Vergara MN, et al. Exp Eye Res. 2013 Apr;109:40-50. doi: 10.1016/j.exer.2013.01.010. Epub 2013 Jan 28. Exp Eye Res. 2013. PMID: 23370269 Free PMC article. - In ovo electroporation in embryonic chick retina.
Islam MM, Doh ST, Cai L. Islam MM, et al. J Vis Exp. 2012 Feb 5;(60):3792. doi: 10.3791/3792. J Vis Exp. 2012. PMID: 22330044 Free PMC article. - Gain- and loss-of-function approaches in the chick embryo.
Sauka-Spengler T, Barembaum M. Sauka-Spengler T, et al. Methods Cell Biol. 2008;87:237-56. doi: 10.1016/S0091-679X(08)00212-4. Methods Cell Biol. 2008. PMID: 18485300 Review.
Cited by
- The Landscape of Non-Viral Gene Augmentation Strategies for Inherited Retinal Diseases.
Toualbi L, Toms M, Moosajee M. Toualbi L, et al. Int J Mol Sci. 2021 Feb 26;22(5):2318. doi: 10.3390/ijms22052318. Int J Mol Sci. 2021. PMID: 33652562 Free PMC article. Review. - Cell type- and stage-specific expression of Otx2 is regulated by multiple transcription factors and _cis_-regulatory modules in the retina.
Chan CSY, Lonfat N, Zhao R, Davis AE, Li L, Wu MR, Lin CH, Ji Z, Cepko CL, Wang S. Chan CSY, et al. Development. 2020 Jul 26;147(14):dev187922. doi: 10.1242/dev.187922. Development. 2020. PMID: 32631829 Free PMC article. - Inhibition of EV71 replication by an interferon-stimulated gene product L3HYPDH.
Liu J, Liu L, Zeng S, Meng X, Lei N, Yang H, Li R, Mu X, Guo X. Liu J, et al. Virus Res. 2024 Apr;342:199336. doi: 10.1016/j.virusres.2024.199336. Epub 2024 Feb 13. Virus Res. 2024. PMID: 38342315 Free PMC article. - A Novel Mucosal Adjuvant System for Immunization against Avian Coronavirus Causing Infectious Bronchitis.
Chandrasekar SS, Kingstad-Bakke B, Wu CW, Suresh M, Talaat AM. Chandrasekar SS, et al. J Virol. 2020 Sep 15;94(19):e01016-20. doi: 10.1128/JVI.01016-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32669327 Free PMC article. - PRISM offers a comprehensive genomic approach to transcription factor function prediction.
Wenger AM, Clarke SL, Guturu H, Chen J, Schaar BT, McLean CY, Bejerano G. Wenger AM, et al. Genome Res. 2013 May;23(5):889-904. doi: 10.1101/gr.139071.112. Epub 2013 Feb 4. Genome Res. 2013. PMID: 23382538 Free PMC article.
References
- Livesey, F. J., Furukawa, T., Steffen, M. A., Church, G. M. & Cepko, C. L. (2000) Curr. Biol. 10, 301–310. - PubMed
- Farjo, R., Yu, J., Othman, M. I., Yoshida, S., Sheth, S., Glaser, T., Baehr, W. & Swaroop, A. (2002) Vision Res. 42, 463–470. - PubMed
- Blackshaw, S., Fraioli, R. E., Furukawa, T. & Cepko, C. L. (2001) Cell 107, 579–589. - PubMed
- Turner, D. L. & Cepko, C. L. (1987) Nature 328, 131–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials