AP-1 recruitment to VAMP4 is modulated by phosphorylation-dependent binding of PACS-1 - PubMed (original) (raw)
Comparative Study
AP-1 recruitment to VAMP4 is modulated by phosphorylation-dependent binding of PACS-1
Ina Hinners et al. EMBO Rep. 2003 Dec.
Abstract
The R-SNARE VAMP4, which contains a dileucine motif, binds to the AP-1 (adaptor protein-1) subunit mu 1a, but not mu 1b, or the GGAs (Golgi-associated gamma ear containing ARF binding proteins). Serine 20 and leucines 25,26 are essential for this binding. AP-1 association with VAMP4 is enhanced when serine 30, in an acidic cluster, is phosphorylated by casein kinase 2. This phosphorylation-dependent modulation of AP-1 binding is mediated by PACS-1 (phosphofurin acidic cluster sorting protein). Ablation of both the dileucine motif and serine 30 results in a dramatic mislocalization of VAMP4 in the regulated secretory pathway in AtT20 cells. A dominant-negative PACS-1, which binds acidic clusters but not AP-1, also causes mislocalization of VAMP4. Our data support a model whereby phosphorylation-dependent recruitment of PACS-1 enhances AP-1 association to cargo, and suggest that efficient retrieval depends on the formation of a complex between cargo, such as VAMP4, AP-1 and PACS-1.
Figures
Figure 1
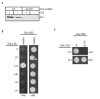
Binding of VAMP4 to AP-1 is modulated by CK2. (A) VAMP4 contains a dileucine motif surrounded by two acidic clusters (boxed). (B) GST-syntaxin 6 or -VAMP4 were pretreated with or without CK2, bound to glutathione beads and incubated with BAMC. AP-1 binds GST-VAMP4, but not syntaxin 6, and binding is enhanced by CK2 phosphorylation. AP-2 does not bind either syntaxin 6 or VAMP4. (C) Immobilized his-GGA-VHS domains were incubated with GST-CI-MPR or -VAMP4. GGA1 bound to the CI-MPR, while no binding of GGA1, 2 or 3-VHS to VAMP4 was detected (data not shown). (D) Yeast was co-transformed with VAMP42–60 and the GGA1–3-VHS domain, and histidine-dependent growth was assessed. The GGAs do not bind to VAMP42–60, while specific GGA interaction with CI-MPR was detected.
Figure 2
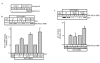
VAMP4 binds exclusively to the μ1a subunit of AP-1 via its dileucine motif. (A) GST-VAMP4 or the LL/VV mutant were pretreated with or without CK2, bound to glutathione beads, incubated with BAMC and processed as in Fig. 1B. Mutations in the dileucine motif abolish AP-1 binding. (B) Yeast was co-transformed with VAMP42–60 and adaptins. A direct interaction of VAMP42–60 with the μ1a subunit of AP-1, but not with other adaptins, was observed. (C) Mutation of the dileucines in VAMP42–60 abolishes μ1a interaction.
Figure 3
Serine 30 in VAMP4 is phosphorylated by CK2. (A) GST-VAMP4 or mutants were incubated with CK2 and [γ32P]-GTP. CK2 phosphorylates wt, LL/VV and S20A, but not S30A VAMP4. In the first two lanes, the asterisk indicates a band present in the CK2 preparation. (B,C) GST-VAMP4 or VAMP4 mutants were incubated and processed as in Fig. 1B. AP-1 binds to S20A only after CK2 phosphorylation. Efficient AP-1 binding occurs to S30D in a CK2-independent fashion, while the S30A mutant binds weakly. The lower panel shows the quantitation of the AP-1 binding to wt VAMP4 and S30A and S30D mutants in the presence or absence of CK2. (D) Reconstitution of the binary VAMP4/AP-1 complex. GST-VAMP4 proteins bound to glutathione beads were incubated with 1 μg of purified AP-1. wt VAMP4, S30A and S30D show equivalent amounts of AP-1 binding, while binding to LL/VV, LL/VV S30A and LL/VV S30 is abolished.
Figure 4
Phosphoserine 30 modulates AP-1 binding to VAMP4 via PACS-1. (A) AtT20 cells were infected with either wt or HA-PACS-1 vaccinia virus and used for immunoprecipitation with anti-VAMP4 antibody. HA-PACS-1 can be detected in the VAMP4 immunoprecipitates. (B) GST-VAMP4 was incubated with or without CK2, bound to glutathione beads and incubated with PACS-1. PACS-1 binding to wt or the LL/VV VAMP4 is enhanced by the presence of CK2; the S30D mutant shows a CK2-independent binding comparable to phosphorylated VAMP4. The lower panel shows quantitation of the PACS-1 binding to wt VAMP4 and mutants in the presence or absence of CK2. (C) Formation of the ternary VAMP4/PACS-1/AP-1 complex using GST-VAMP4, PACS-1 and purified AP-1. Addition of PACS-1 protein to the VAMP4 LL/VV S30D protein enhances binding of AP-1 compared to VAMP4 LL/VV S30A protein. Quantitation (lower panel) of AP-1 binding to the VAMP4/PACS-1 complex shows a PACS-1-dependent increase.
Figure 5
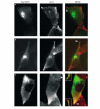
Expression of PACS-1 admut causes mislocalization of VAMP4. AtT20 cells were transfected with Flag-VAMP4 and after 60 h infected with either (A) wt, (B) HA-PACS-1 or (C) PACS-1 admut vaccinia virus. Flag-VAMP4 panels (green, detected with M2 ab) show the localization of VAMP4, ACTH panels (red, detected with anti-POMC ab) show the localization of POMC and ACTH, and MERGE panels show the merge of Flag and ACTH. Expression of PACS-1 admut (see the arrowhead in (C)) but not PACS-1 (see arrowheads in (A, B)) causes mislocalization of Flag-VAMP4 to the ACTH-positive MSGs in the tips of the cells. The insets in (C) show double labelling of VAMP4 and ACTH in two other tips.
Figure 6
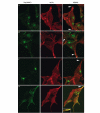
Sorting of VAMP4 in AtT20 cells requires both AP-1 and PACS-1 binding sites. The localization of transfected Flag-tagged (A) wt VAMP4, (B) VAMP4 LL/VV, (C) VAMP4 S30A or (D) VAMP4 LL/VV S30A was compared to endogenous ACTH in AtT20 cells. The left panels show the Flag tag (green, M2 ab), the centre panels show ACTH (red, POMC ab) and the right panels show the MERGE of the two images. The wt, LL/VV and S30A proteins remain predominantly in a perinuclear region, and are not found in the tips (arrowheads in (A–C)) with ACTH. Mutation of both the dileucine motif and the PACS-1 binding motif results in mislocalization of VAMP4 to ACTH-positive MSGs found in the tips of the cells (arrowhead in (D)). The inset in (C) MERGE shows double labelling of VAMP4 and ACTH in another tip.
Similar articles
- PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic.
Crump CM, Xiang Y, Thomas L, Gu F, Austin C, Tooze SA, Thomas G. Crump CM, et al. EMBO J. 2001 May 1;20(9):2191-201. doi: 10.1093/emboj/20.9.2191. EMBO J. 2001. PMID: 11331585 Free PMC article. - Identification of acidic dileucine signals in LRP9 that interact with both GGAs and AP-1/AP-2.
Doray B, Knisely JM, Wartman L, Bu G, Kornfeld S. Doray B, et al. Traffic. 2008 Sep;9(9):1551-62. doi: 10.1111/j.1600-0854.2008.00786.x. Epub 2008 Jul 9. Traffic. 2008. PMID: 18627575 Free PMC article. - Polarized sorting of the copper transporter ATP7B in neurons mediated by recognition of a dileucine signal by AP-1.
Jain S, Farías GG, Bonifacino JS. Jain S, et al. Mol Biol Cell. 2015 Jan 15;26(2):218-28. doi: 10.1091/mbc.E14-07-1177. Epub 2014 Nov 5. Mol Biol Cell. 2015. PMID: 25378584 Free PMC article. - [Regulatory mechanisms of the clathrin adaptor molecules AP-1 and GGAs].
Kametaka S, Bonifacino JS. Kametaka S, et al. Tanpakushitsu Kakusan Koso. 2008 Dec;53(16 Suppl):2046-52. Tanpakushitsu Kakusan Koso. 2008. PMID: 21038583 Review. Japanese. No abstract available. - Cargo Sorting at the _trans_-Golgi Network for Shunting into Specific Transport Routes: Role of Arf Small G Proteins and Adaptor Complexes.
Tan JZA, Gleeson PA. Tan JZA, et al. Cells. 2019 Jun 3;8(6):531. doi: 10.3390/cells8060531. Cells. 2019. PMID: 31163688 Free PMC article. Review.
Cited by
- AP180-mediated trafficking of Vamp7B limits homotypic fusion of Dictyostelium contractile vacuoles.
Wen Y, Stavrou I, Bersuker K, Brady RJ, De Lozanne A, O'Halloran TJ. Wen Y, et al. Mol Biol Cell. 2009 Oct;20(20):4278-88. doi: 10.1091/mbc.e09-03-0243. Epub 2009 Aug 19. Mol Biol Cell. 2009. PMID: 19692567 Free PMC article. - VAMP4 Is an Essential Cargo Molecule for Activity-Dependent Bulk Endocytosis.
Nicholson-Fish JC, Kokotos AC, Gillingwater TH, Smillie KJ, Cousin MA. Nicholson-Fish JC, et al. Neuron. 2015 Dec 2;88(5):973-984. doi: 10.1016/j.neuron.2015.10.043. Epub 2015 Nov 19. Neuron. 2015. PMID: 26607000 Free PMC article. - A PACS-1, GGA3 and CK2 complex regulates CI-MPR trafficking.
Scott GK, Fei H, Thomas L, Medigeshi GR, Thomas G. Scott GK, et al. EMBO J. 2006 Oct 4;25(19):4423-35. doi: 10.1038/sj.emboj.7601336. Epub 2006 Sep 14. EMBO J. 2006. PMID: 16977309 Free PMC article. - Monitoring activity-dependent bulk endocytosis with the genetically-encoded reporter VAMP4-pHluorin.
Nicholson-Fish JC, Smillie KJ, Cousin MA. Nicholson-Fish JC, et al. J Neurosci Methods. 2016 Jun 15;266:1-10. doi: 10.1016/j.jneumeth.2016.03.011. Epub 2016 Mar 23. J Neurosci Methods. 2016. PMID: 27015791 Free PMC article. - Cholesterol accumulation increases insulin granule size and impairs membrane trafficking.
Bogan JS, Xu Y, Hao M. Bogan JS, et al. Traffic. 2012 Nov;13(11):1466-80. doi: 10.1111/j.1600-0854.2012.01407.x. Epub 2012 Sep 13. Traffic. 2012. PMID: 22889194 Free PMC article.
References
- Austin C., Hinners I. & Tooze S.A. ( 2000) Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem., 275, 21862–21869. - PubMed
- Boman A.L. ( 2001) GGA proteins: new players in the sorting game. J. Cell Sci., 114, 3413–3418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI049793/AI/NIAID NIH HHS/United States
- AI49793/AI/NIAID NIH HHS/United States
- R01 AI048585/AI/NIAID NIH HHS/United States
- R01 DK037274/DK/NIDDK NIH HHS/United States
- AI48585/AI/NIAID NIH HHS/United States
- DK37274/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous