Hrs mediates downregulation of multiple signalling receptors in Drosophila - PubMed (original) (raw)
Hrs mediates downregulation of multiple signalling receptors in Drosophila
Gáspár Jékely et al. EMBO Rep. 2003 Dec.
Abstract
Endocytosis and subsequent lysosomal degradation of activated signalling receptors can attenuate signalling. Endocytosis may also promote signalling by targeting receptors to specific compartments. A key step regulating the degradation of receptors is their ubiquitination. Hrs/Vps27p, an endosome-associated, ubiquitin-binding protein, affects sorting and degradation of receptors. Drosophila embryos mutant for hrs show elevated receptor tyrosine kinase (RTK) signalling. Hrs has also been proposed to act as a positive mediator of TGF-beta signalling. We find that Drosophila epithelial cells devoid of Hrs accumulate multiple signalling receptors in an endosomal compartment with high levels of ubiquitinated proteins: not only RTKs (EGFR and PVR) but also Notch and receptors for Hedgehog and Dpp (TGF-beta related). Hrs is not required for Dpp signalling. Instead, loss of Hrs increases Dpp signalling and the level of the type-I receptor Thickveins (Tkv). Finally, most hrs-dependent receptor turnover appears to be ligand independent. Thus, both active and inactive signalling receptors are targeted for degradation in vivo and Hrs is required for their removal.
Figures
Figure 1
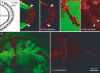
Accumulation of ubiquitinated proteins in hrs mutant cells. Ubiquitinated proteins accumulate intracellularly in hrs mutant cells. Mosaic egg chambers and wing discs were stained with an antibody against ubiquitinated proteins (red). (A) Schematic diagram of a transverse section through the oocyte and the follicle cells, as shown in (B). (C) Optical section in the plane of the follicular epithelium.hrs mutant cells are marked by the absence of GFP (green) in both ovarian follicle cells (B, C) and wing discs (D). Some ubiquitinated proteins appear to be at the cell cortex (arrows in (C)). Arrowheads in (B) indicate the boundary between mutant and wild-type cells.
Figure 2
Ubiquitinated proteins accumulate in enlarged endosomes in_hrs_ mutant cells. Egg chambers expressing GFP-2xFYVE (A, C) or GFP-Rab5 (B) (green) and carrying patches of hrs mutant follicle cells were stained with an antibody against ubiquitinated proteins (red). In (A) and (B) all cells shown are hrs mutant, and in (C) the boundary between mutant and wild-type cells is indicated with a dashed line. hrs mutant cells can be detected by distinctive staining with the ubiquitinated protein antibody. Note the increased staining with the endosomal marker (FYVE) in mutant cells relative to neighbouring cells. Phalloidin-stained F-actin (blue) outlines cells in the overlay to the right.
Figure 3
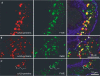
Colocalization of signalling receptors and ubiquitinated proteins in_hrs_ mutant cells. Egg chambers with patches of hrs mutant follicle cells were stained with an antibody against ubiquitinated protein (green) and antibodies against the following specific proteins (red): PVR (A), EGFR (B), Ptc (C), Smo (D), Tkv (E), Notch (F) and DE-cadherin (G). Notch could not be analysed for colocalization with the ubiquitinated protein due to antibody incompatibility. Instead, labelled phalloidin (green) is used to mark cell outlines in (F) and (G). The overlap between the signals is yellow in the merged images (right). The boundary between hrs mutant and wild-type cells is indicated with arrowheads (A) or with dashed lines (B–G). Mutant cells are marked by the absence of GFP (blue) in the merged images. (A, D, F, G) Similar transverse sections through the egg chamber, with the apical side of the follicle cells towards the oocyte (bottom of image). (B, C,E) More oblique sections through the follicular epithelium.
Figure 4
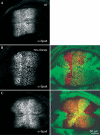
Role of Hrs in Dpp signalling and Tkv trafficking in follicle cells. (A) Schematic cross-section of stage 10 egg chamber. A small and a large stippled box indicate areas shown in (B, C) and (D,G, H), respectively. (D, H) Surface views of the epithelium, as are (E) and (F). Anterior follicle cells (red in (A)) express Dpp and Dpp-lacZ (see also (B)). P-MAD staining visualizes Dpp signalling activity in the cells expressing Dpp and in adjacent cells (C). The Dpp receptor Tkv is detected in all follicle cells**(D). Wild-type (C, D) and hrs mosaic (E–H**) stage 10 egg chambers stained with antibodies (red in double-staining) against β-galactosidase (B), P-MAD (C,E, F) or Tkv (D, G, H). (B) and (C) are also stained with phalloidin (green). Mutant cells are marked by the absence of GFP (blue) in (E–H). The boundary between_hrs_ mutant and wild-type cells is also indicated with an arrowhead (G) or a dashed line (H). In (E) no follicle cells are mutant, and in (F) all follicle cells are mutant for hrs. The double-headed arrow (E, F) indicates the P-MAD positive domain, which is expanded when cells are mutant for hrs.
Figure 5
Dpp target gene expression is increased in hrs mutant wing disc cells. Wild-type (A) and hrs mosaic (B, C) wing discs were stained with an antibody against Spalt (red in the merged images). hrs mutant cells are marked by the absence of GFP (green). Dpp is expressed in the middle of the disc and induces Spalt expression in a broad domain. Note the slight increase in Spalt expression in hrs mutant cells within the normal expression domain (B) and the expansion of the Spalt expression domain when hrs mutant cells are at the edge of this domain ((C), most clear in the middle of the disc, to the right).
Similar articles
- The Hrs/Stam complex acts as a positive and negative regulator of RTK signaling during Drosophila development.
Chanut-Delalande H, Jung AC, Baer MM, Lin L, Payre F, Affolter M. Chanut-Delalande H, et al. PLoS One. 2010 Apr 21;5(4):e10245. doi: 10.1371/journal.pone.0010245. PLoS One. 2010. PMID: 20422006 Free PMC article. - TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation.
Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. Lu Q, et al. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7626-31. doi: 10.1073/pnas.0932599100. Epub 2003 Jun 11. Proc Natl Acad Sci U S A. 2003. PMID: 12802020 Free PMC article. - Hrs and endocytic sorting of ubiquitinated membrane proteins.
Raiborg C, Stenmark H. Raiborg C, et al. Cell Struct Funct. 2002 Dec;27(6):403-8. doi: 10.1247/csf.27.403. Cell Struct Funct. 2002. PMID: 12576633 Review. - Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila.
Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Lloyd TE, et al. Cell. 2002 Jan 25;108(2):261-9. doi: 10.1016/s0092-8674(02)00611-6. Cell. 2002. PMID: 11832215 - The interface of receptor trafficking and signalling.
Clague MJ, Urbé S. Clague MJ, et al. J Cell Sci. 2001 Sep;114(Pt 17):3075-81. doi: 10.1242/jcs.114.17.3075. J Cell Sci. 2001. PMID: 11590234 Review.
Cited by
- Endocytosis and signalling: intertwining molecular networks.
Sorkin A, von Zastrow M. Sorkin A, et al. Nat Rev Mol Cell Biol. 2009 Sep;10(9):609-22. doi: 10.1038/nrm2748. Nat Rev Mol Cell Biol. 2009. PMID: 19696798 Free PMC article. Review. - At the crossroads of polarity, proliferation and apoptosis: the use of Drosophila to unravel the multifaceted role of endocytosis in tumor suppression.
Vaccari T, Bilder D. Vaccari T, et al. Mol Oncol. 2009 Aug;3(4):354-65. doi: 10.1016/j.molonc.2009.05.005. Epub 2009 Jun 6. Mol Oncol. 2009. PMID: 19560990 Free PMC article. Review. - The clathrin-binding motif and the J-domain of Drosophila Auxilin are essential for facilitating Notch ligand endocytosis.
Kandachar V, Bai T, Chang HC. Kandachar V, et al. BMC Dev Biol. 2008 May 8;8:50. doi: 10.1186/1471-213X-8-50. BMC Dev Biol. 2008. PMID: 18466624 Free PMC article. - Maintenance of glia in the optic lamina is mediated by EGFR signaling by photoreceptors in adult Drosophila.
Lee YM, Sun YH. Lee YM, et al. PLoS Genet. 2015 Apr 24;11(4):e1005187. doi: 10.1371/journal.pgen.1005187. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25909451 Free PMC article. - Comparative Analysis of Transmembrane Regulators of the Filamentous Growth Mitogen-Activated Protein Kinase Pathway Uncovers Functional and Regulatory Differences.
Adhikari H, Caccamise LM, Pande T, Cullen PJ. Adhikari H, et al. Eukaryot Cell. 2015 Sep;14(9):868-83. doi: 10.1128/EC.00085-15. Epub 2015 Jun 26. Eukaryot Cell. 2015. PMID: 26116211 Free PMC article.
References
- Bilodeau P.S., Urbanowski J.L., Winistorfer S.C. & Piper R.C. ( 2002) The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol., 4, 534–539. - PubMed
- Christoforidis S., Miaczynska M., Ashman K., Wilm M., Zhao L., Yip S.C., Waterfield M.D., Backer J.M. & Zerial M. ( 1999) Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol., 1, 249–252. - PubMed
- Denef N., Neubuser D., Perez L. & Cohen S.M. ( 2000) Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell, 102, 521–531. - PubMed
- Duchek P., Somogyi K., Jékely G., Beccari S. & Rørth P. ( 2001) Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell, 107, 17–26. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous