Degradation of origin recognition complex large subunit by the anaphase-promoting complex in Drosophila - PubMed (original) (raw)
Degradation of origin recognition complex large subunit by the anaphase-promoting complex in Drosophila
Marito Araki et al. EMBO J. 2003.
Abstract
The initiation of DNA synthesis is thought to occur at sites bound by a heteromeric origin recognition complex (ORC). Previously, we have shown that in Drosophila, the level of the large subunit, ORC1, is modulated during cell cycle progression and that changes in ORC1 concentration alter origin utilization during development. Here, we investigate the mechanisms underlying cell cycle-dependent degradation of ORC1. We show that signals in the non-conserved N-terminal domain of ORC1 mediate its degradation upon exit from mitosis and in G1 phase by the anaphase-promoting complex (APC) in vivo. Degradation appears to be the result of direct action of the APC, as the N-terminal domain is ubiquitylated by purified APC in vitro. This regulated proteolysis is potent, sufficient to generate a normal temporal distribution of protein even when transcription of ORC1 is driven by strong constitutive promoters. These observations suggest that in Drosophila, ORC1 regulates origin utilization much as does Cdc6 in budding yeast.
Figures
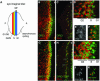
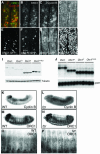
Fig. 1. Persistence of ORC1 into M phase. (A) Schematic of the synchronous cell cycle transition in the eye imaginal disc. As the morphogenetic furrow (MF, hereinafter marked with an arrowhead) sweeps from posterior (P) to anterior (A), most cells undergo a synchronous transition and then enter a prolonged G1/G0 phase. (Note that some cells behind the MF are in a prolonged G2 arrest, as shown in Figure 3.) Ahead of the furrow and in the attached antennal disc, cells cycle asynchronously. (B–K) Confocal images of eye imaginal discs near the MF (arrowhead). Endogenous ORC1 (B–F) and ORC1–GFP expressed under ORC1 transcriptional control (G–K), and CycB or PH3, as indicated. Interphase nuclei are visible in optical sections through the middle of the disc (B, D, G and I), whereas mitotic nuclei are visible in apical optical sections (C, E, F, H, J and K). Arrows in (H) are examples of late telophase nuclei (see also Figure 3K–M).
Fig. 2. Absence of ORC1 in G1 phase. FACS analysis of dissociated imaginal disc cells from transgenic animals expressing ORC1–GFP under ORC1 transcriptional control. The proportion of G1 cells in eye antennal discs is higher than in wing discs, due to the contribution of terminally differentiating cells behind the morphogenetic furrow.
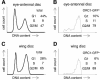
Fig. 3. Regulated proteolysis generates a normal temporal distribution of ORC1 protein even upon constitutive ORC1 transcription. Expression of various proteins under control of the GMR promoter (A–C, E–H, K and L), which is active in all cells posterior to the MF (arrowhead) in the eye disc, or the engrailed (en) promoter (I and J), which is active in all cells of the posterior compartment of the wing disc. High magnification views of the area of the eye disc boxed in (A) reveal that cells with high levels of ORC1–GFP are CycB positive with no BrdU incorporation (not shown), and therefore in G2 (B–D). FACS analysis reveals that ORC1–GFP is depleted in G1 phase eye (G and H) and wing (I and J) imaginal disc cells, even if transcription of ORC1–GFP is driven constitutively. High magnification views of cells immediately posterior to the MF reveal co-localization of ORC1–GFP and CycB following nuclear envelope breakdown and the presence of late telophase cells with paired nuclei bearing high levels of ORC1–GFP but no significant CycB (K–M).
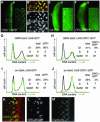
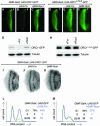
Fig. 4. Signals that mediate regulated proteolysis of ORC1 reside in its non-conserved N-terminal domain. (A) Schema of the deletion derivatives analyzed in (B–K). Expression of various ORC1 derivatives in the eye disc under GMR promoter control (B–E, each with a high magnification view inset), also analyzed by western blot (F), northern blot (G) and dissociation into single cells followed by FACS (H–K). The gels in (F) and (G) were re-probed with the loading controls shown at the bottom of each blot. Analysis of western blots reveals that the steady-state level of ORC1 or ORC1N is ∼25% that of ORC1C. Since these samples are homogenates of cells with low and high levels of protein, it underestimates the extent of regulation per cell (probably by a factor of ∼2, based on the cell cycle distributions of H–K).
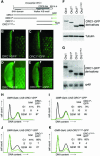
Fig. 5. APC-dependent degradation of ORC1 in embryonic cells. Confocal images of epithelial cells just ventral to the leading edge in stage 12–13 embryos (A–H). At this stage, most cells are already in G1 of cycle 17 and only a minority of cells are in S or G2 of cycle 16, with high levels of both ORC1 and CycB (A–C). In (D–H), transcription of various proteins (as indicated) is driven by the constitutive Actin5C promoter. The few cells with high levels of ORC1–GFP or ORC1N–GFP also contain appreciable CycB and therefore are still in G2 of cycle 16 (not shown). We used northern blots (J) to select transgenic UAS lines that direct transcription of essentially identical levels of each gene, and then analyzed the steady-state level of each ORC1–GFP derivative by western blot (I). This analysis reveals that the steady-state level of ORC1C and ORC1C–NLS is 4- to 6-fold higher than ORC1–GFP and 6- to 9-fold higher than ORC1N. As in Figure 4, this analysis underestimates the extent of regulation per cell. Transmitted light microscopy reveals accumulation of CycB (K versus L) and ORC1 (M–P) in essentially every epithelial cell of fzr mutant embryos. Note that differential accumulation in internal tissues (primarily midgut) partially obscures accumulation in epithelial cells.
Fig. 6. APC-mediated degradation of ORC1–GFP in imaginal cells. Co-expression of Fzr, which stimulates APC-dependent substrate degradation, significantly destabilizes ORC1–GPF in the eye disc (B versus A), whereas co-expression of Rca1, an inhibitor of Fzr, has the opposite effect (C). No effect was seen on the stability of the unregulated C-terminal domain of ORC1 (D–F). Western blots of the same disc samples are shown in (G) and (H). The distribution of BrdU incorporated during a brief pulse reveals that misexpression of neither Fzr nor Rca1 drives cells ectopically into S phase (I–K), although we see a slight effect similar to that reported (Dong et al., 1997) in the latter case. FACS analysis (L and M) reveals that misexpression of Fzr causes a modest accumulation of G2 cells (at the expense of the G1 population), whereas misexpression of Rca1 has no effect on the profile of cells in the posterior of the eye disc where the GMR promoter is active. Note that these experiments were performed in the absence of UAS-ORC1–GFP transgenes, but that expression of ORC1–GFP derivatives alone does not alter cycling of eye disc cells (Figures 2–4).
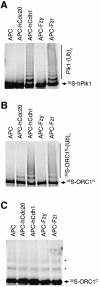
Fig. 7. Ubiquitylation of ORC1N by purified APC in vitro. Human Plk1 (A), Drosophila ORC1N (B) and Drosophila ORC1C (C) were incubated with purified Xenopus APC alone (lane 1 of each panel), or APC supplemented with the activator indicated above each lane. Ubiquitylation was detected by the generation of a higher molecular weight ladder following electrophoresis and autoradiography. The asterisks in (C) mark non-specifically ubiquitylated forms of the ORC1C substrate independent of active APC.
Fig. 8. Model of our current understanding of the temporal distribution of ORC1 in fly, human and either budding or fission yeast cells. Drosophila Cdc6 is uncharacterized and therefore omitted.
Similar articles
- A novel motif governs APC-dependent degradation of Drosophila ORC1 in vivo.
Araki M, Yu H, Asano M. Araki M, et al. Genes Dev. 2005 Oct 15;19(20):2458-65. doi: 10.1101/gad.1361905. Epub 2005 Sep 29. Genes Dev. 2005. PMID: 16195415 Free PMC article. - Function of the origin recognition complex 1 (ORC1) outside DNA replication in Drosophila.
Park SY, Asano M. Park SY, et al. Cell Cycle. 2011 Nov 15;10(22):3957-63. doi: 10.4161/cc.10.22.18133. Epub 2011 Nov 15. Cell Cycle. 2011. PMID: 22071690 - An orc1 allele with a mutated APC motif is female sterile with amplification defects.
Park SY, Asano M. Park SY, et al. Cell Cycle. 2012 Aug 1;11(15):2828-32. doi: 10.4161/cc.21168. Epub 2012 Aug 1. Cell Cycle. 2012. PMID: 22801552 - Subunits and substrates of the anaphase-promoting complex.
Peters JM. Peters JM. Exp Cell Res. 1999 May 1;248(2):339-49. doi: 10.1006/excr.1999.4443. Exp Cell Res. 1999. PMID: 10222126 Review. - Control of mitotic transitions by the anaphase-promoting complex.
Fang G, Yu H, Kirschner MW. Fang G, et al. Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1583-90. doi: 10.1098/rstb.1999.0502. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582244 Free PMC article. Review.
Cited by
- Mechanisms of ubiquitin transfer by the anaphase-promoting complex.
Matyskiela ME, Rodrigo-Brenni MC, Morgan DO. Matyskiela ME, et al. J Biol. 2009;8(10):92. doi: 10.1186/jbiol184. J Biol. 2009. PMID: 19874575 Free PMC article. Review. - Regulation of Mammalian DNA Replication via the Ubiquitin-Proteasome System.
Abbas T, Dutta A. Abbas T, et al. Adv Exp Med Biol. 2017;1042:421-454. doi: 10.1007/978-981-10-6955-0_19. Adv Exp Med Biol. 2017. PMID: 29357069 Free PMC article. Review. - The Non-Canonical Role of Aurora-A in DNA Replication.
Tsunematsu T, Arakaki R, Yamada A, Ishimaru N, Kudo Y. Tsunematsu T, et al. Front Oncol. 2015 Aug 25;5:187. doi: 10.3389/fonc.2015.00187. eCollection 2015. Front Oncol. 2015. PMID: 26380219 Free PMC article. Review. - Nonreplicative functions of the origin recognition complex.
Popova VV, Brechalov AV, Georgieva SG, Kopytova DV. Popova VV, et al. Nucleus. 2018;9(1):460-473. doi: 10.1080/19491034.2018.1516484. Nucleus. 2018. PMID: 30196754 Free PMC article. Review. - A novel motif governs APC-dependent degradation of Drosophila ORC1 in vivo.
Araki M, Yu H, Asano M. Araki M, et al. Genes Dev. 2005 Oct 15;19(20):2458-65. doi: 10.1101/gad.1361905. Epub 2005 Sep 29. Genes Dev. 2005. PMID: 16195415 Free PMC article.
References
- Aparicio O.M., Weinstein,D.M. and Bell,S.P. (1997) Components and dynamics of DNA replication complexes in S.cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell, 91, 59–69. - PubMed
- Asano M., Nevins,J.R. and Wharton,R.P. (1996) Ectopic E2F expression induces S phase and apoptosis in Drosophila imaginal discs. Genes Dev., 10, 1422–1432. - PubMed
- Bell S.P. (1995) Eukaryotic replicators and associated protein complexes. Curr. Opin. Genet. Dev., 5, 162–167. - PubMed
- Bell S.P. (2002) The origin recognition complex: from simple origins to complex functions. Genes Dev., 16, 659–672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases