Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells - PubMed (original) (raw)
Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells
Brent Berwin et al. EMBO J. 2003.
Abstract
gp96 (GRP94) elicits antigen-presenting cell (APC) activation and can direct peptides into the cross- presentation pathways of APC. These responses arise through interactions of gp96 with Toll-like (APC activation) and endocytic (cross-presentation) receptors of APC. Previously, CD91, the alpha2-macroglobulin receptor, was identified as the heat shock/chaperone protein receptor of APC. Recent data indicates, however, that inhibition of CD91 ligand binding does not alter gp96 recognition and uptake. Furthermore, CD91 expression is not itself sufficient for gp96 binding and internalization. We now report that scavenger receptor class-A (SR-A), a prominent scavenger receptor of macrophages and dendritic cells, serves a primary role in gp96 and calreticulin recognition and internalization. gp96 internalization and peptide re-presentation are inhibited by the SR-A inhibitory ligand fucoidin, although fucoidin was without effect on alpha2-macroglobulin binding or uptake. Ectopic expression of SR-A in HEK 293 cells yielded gp96 recognition and uptake activity. In addition, macrophages derived from SR-A-/- mice were substantially impaired in gp96 binding and uptake. These data identify new roles for SR-A in the regulation of cellular responses to heat shock proteins.
Figures
Fig. 1. The SR-A inhibitory ligand fucoidin blocks binding of gp96 and calreticulin to peritoneal macrophages. (A) Fucoidin, but not chondroitin sulfate (75 µg/ml), inhibits the binding of fluorescein(Fl)-conjugated gp96 to elicited peritoneal macrophages. Fucoidin (75 µg/ml) or chondroitin sulfate (75 µg/ml) was added to cultures of adherence-selected peritoneal murine macrophages in the presence of 8 µg/ml Fl–gp96 for 30 min on ice. Cells were subsequently washed, fixed and processed for flow cytometry. (B) Fucoidin inhibits gp96 binding to elicited peritoneal macrophages in a dose-dependent manner. Fl–gp96 binding to peritoneal macrophages was performed in the presence of increasing concentrations of fucoidin, the cells fixed, and analyzed by flow cytometry. (C) Fucoidin (250 µg/ml) inhibits the binding of fluorescein-conjugated calreticulin (20 µg/ml) to elicited peritoneal macrophages. Experiments were conducted as in (A). (D) Uptake of Alexa-conjugated calreticulin is partially inhibited by non-conjugated gp96 or unconjugated calreticulin (CRT). Cellular internalization of Alexa-conjugated calreticulin was assayed in the presence of increasing concentrations of non-conjugated gp96 or calreticulin. The partial inhibition of CRT internalization observed with protein ligands is contrasted by that seen with fucoidin (filled triangles). (E) The binding of the CD91 ligand α2M to elicited peritoneal macrophages is not inhibited by fucoidin. Alexa-conjugated α2M binding to peritoneal macrophages was performed in the presence of a 10-fold molar excess of fucoidin, as in (A). Solid lines in (A), (C) and (E) represent autofluorescence.
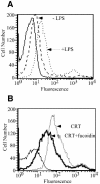
Fig. 2. Endotoxin stimulation of RAW264.7 macrophages elicits the upregulation of a fucoidin-sensitive gp96/CRT receptor. (A) RAW264.7 macrophages were treated with 10 ng/ml LPS for 16 h. Following treatment, cells were washed and the binding of fluorescein-conjugated CRT examined, as described in the legend to Figure 1. (B) CRT- binding to the up-regulated receptor is fucoidin-sensitive. As in (A), LPS stimulated RAW264.7 macrophages were incubated with 5 µg/ml fl–CRT in the presence or absence of 25 µg/ml fucoidin and binding histograms determined by flow cytometry.
Fig. 3. SR-A expression is sufficient for the binding and internalization of gp96 and CRT. HEK 293 cells bearing SR-AII under control of a tetracycline inducible promoter (HEK-SRATET) were examined for gp96 and CRT uptake in the uninduced and induced state (see Materials and methods). Both populations of cells were incubated with 12 µg/ml Fl–gp96 (A), Fl–CRT (B) or 2.5 µg/ml Alexa488–AcLDL (C) at 37°C to assess receptor-mediated uptake. Where indicated, 75 µg/ml fucoidin was included to block SR-A-dependent uptake. Following the uptake period, cells were trypsinized and chaperone uptake assessed by flow cytometry. (D) Confocal microscopy (and corresponding phase contrast pictures, below) of Fl–gp96 internalization by HEK-SRATET cells (as in A) when uninduced (not expressing SR-A; left), induced (expressing SR-A; middle) and induced, in the presence of fucoidin (right). (E) Uptake of TR–gp96 by HEK-SRATET cells is saturable and SR-A expression-dependent (uninduced cells, diamonds; induced cells, triangles). LPS-stimulated RAW264.7 macrophages (as in Figure 2) were assayed for their ability to internalize 8 µg/ml Fl–gp96 (F) or Fl–α2M (G) in the presence or absence of 175 µg/ml fucoidin, as described in the legend to Figure 1. Uptake was analyzed by flow cytometry. Solid lines in (A), (B), (F) and (G) are autofluorescence.
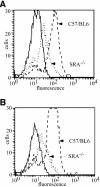
Fig. 4. SR-A–/– macrophages are deficient in gp96 and CRT binding. Elicited peritoneal macrophages from C57/BL6 and SR-A–/– (C57/BL6 background) mice were assayed for their ability to bind and internalize gp96 and CRT. Cell surface binding experiments were performed for 30 min at 4°C; TR–gp96 (A) TR–CRT (B). Binding of either chaperone to SR-A–/– macrophages was ∼50% that observed for C57/BL6-derived macrophages. Solid lines represent autofluorescence.
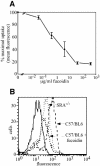
Fig. 5. SR-A mediates gp96 and CRT uptake. (A) Fluorescently labeled gp96 was incubated with elicited peritoneal macrophages for 30 min, 37°C in the presence of the indicated concentrations of fucoidin. Subsequently cells were washed fixed, and chaperone uptake assayed by FACS analysis. (B) SR-A–/– macrophages were tested for their ability to endocytose CRT, relative to wild-type (C57Bl/6-derived) macrophages. Analogous to the experiments performed in (A), chaperone internalization experiments were performed for 7 min at 37°C. SR-A–/– macrophages display ∼50% of the receptor-mediated chaperone uptake of control macrophages. Similar results were observed for gp96.
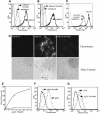
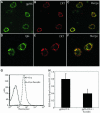
Fig. 6. SR-A directs gp96 and CRT to an FcR+ endosomal compartment competent for the re-presentation of gp96-associated peptides. (A–F) Fluorescein-labeled gp96 (A and C) or IgG (D and F) and TR–CRT (B,C, E and F) were allowed to bind to the surface of macrophages on ice and the cells were subsequently washed to remove unbound ligand. Cells were then warmed to 37°C for 10 min, to allow ligand internalization, and the cells subsequently fixed and processed for confocal microscopy. Extensive co-localization (yellow) of receptor-internalized gp96 and IgG with CRT was observed (C,F). (G) 100 nM Ova peptide was incubated with C57/BL6-derived peritoneal macrophages in the presence or absence of 250 µg/ml fucoidin for 2 h. Surface presentation of the Ova peptide in the context of MHC class-I molecules was then assessed with the Kb-, Ova-specific monoclonal antibody 25-D1.16. (H) gp96–Ova peptide covalent complexes (50 µg/ml; ∼250 nM gp96) or unconjugated OVA peptide (100 nM or 1 µM) were incubated with C57/BL6-derived peritoneal macrophages in the presence or absence of 250 µg /ml fucoidin for 30 min. The macrophages were then washed and incubated with B3Z reporter T-cells, which recognize Ova in the context of Kb MHC class-I molecules. B3Z activation was scored as β-galactosidase activity.
Similar articles
- SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin.
Berwin B, Delneste Y, Lovingood RV, Post SR, Pizzo SV. Berwin B, et al. J Biol Chem. 2004 Dec 3;279(49):51250-7. doi: 10.1074/jbc.M406202200. Epub 2004 Sep 14. J Biol Chem. 2004. PMID: 15371419 - Cutting edge: CD91-independent cross-presentation of GRP94(gp96)-associated peptides.
Berwin B, Hart JP, Pizzo SV, Nicchitta CV. Berwin B, et al. J Immunol. 2002 May 1;168(9):4282-6. doi: 10.4049/jimmunol.168.9.4282. J Immunol. 2002. PMID: 11970968 - CD91: a receptor for heat shock protein gp96.
Binder RJ, Han DK, Srivastava PK. Binder RJ, et al. Nat Immunol. 2000 Aug;1(2):151-5. doi: 10.1038/77835. Nat Immunol. 2000. PMID: 11248808 - The heat-shock protein receptors: some answers and more questions.
Binder RJ, Vatner R, Srivastava P. Binder RJ, et al. Tissue Antigens. 2004 Oct;64(4):442-51. doi: 10.1111/j.1399-0039.2004.00299.x. Tissue Antigens. 2004. PMID: 15361121 Review. - An integrated view of the roles and mechanisms of heat shock protein gp96-peptide complex in eliciting immune response.
Li Z, Dai J, Zheng H, Liu B, Caudill M. Li Z, et al. Front Biosci. 2002 Mar 1;7:d731-51. doi: 10.2741/a808. Front Biosci. 2002. PMID: 11861214 Review.
Cited by
- Challenges in the development of an autologous heat shock protein based anti-tumor vaccine.
Reitsma DJ, Combest AJ. Reitsma DJ, et al. Hum Vaccin Immunother. 2012 Aug;8(8):1152-5. doi: 10.4161/hv.21382. Epub 2012 Aug 1. Hum Vaccin Immunother. 2012. PMID: 22854658 Free PMC article. Review. No abstract available. - Cysteine-rich domain of scavenger receptor AI modulates the efficacy of surface targeting and mediates oligomeric Aβ internalization.
Huang FL, Shiao YJ, Hou SJ, Yang CN, Chen YJ, Lin CH, Shie FS, Tsay HJ. Huang FL, et al. J Biomed Sci. 2013 Aug 2;20(1):54. doi: 10.1186/1423-0127-20-54. J Biomed Sci. 2013. PMID: 23915271 Free PMC article. - Both CD4+ and CD8+ T cell epitopes fused to heat shock cognate protein 70 (hsc70) can function to eradicate tumors.
Mizukami S, Kajiwara C, Ishikawa H, Katayama I, Yui K, Udono H. Mizukami S, et al. Cancer Sci. 2008 May;99(5):1008-15. doi: 10.1111/j.1349-7006.2008.00788.x. Epub 2008 Mar 12. Cancer Sci. 2008. PMID: 18341654 Free PMC article. - Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation.
Yi H, Yu X, Gao P, Wang Y, Baek SH, Chen X, Kim HL, Subjeck JR, Wang XY. Yi H, et al. Blood. 2009 Jun 4;113(23):5819-28. doi: 10.1182/blood-2008-11-190033. Epub 2009 Apr 6. Blood. 2009. PMID: 19349620 Free PMC article. - Chaperone-rich cell lysates, immune activation and tumor vaccination.
Zeng Y, Graner MW, Katsanis E. Zeng Y, et al. Cancer Immunol Immunother. 2006 Mar;55(3):329-38. doi: 10.1007/s00262-005-0694-1. Epub 2005 May 11. Cancer Immunol Immunother. 2006. PMID: 15887013 Free PMC article. Review.
References
- Abraham R., Singh,N., Mukhopadhyay,A., Basu,S.K., Bal,V. and Rath,S. (1995) Modulation of immunogenicity and antigenicity of proteins by maleylation to target scavenger receptors on macrophages. J. Immunol., 154, 1–8. - PubMed
- Asea A., Kraeft,S.K., Kurt-Jones,E.A., Stevenson,M.A., Chen,L.B., Finberg,R.W., Koo,G.C. and Calderwood,S.K. (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med., 6, 435–442. - PubMed
- Asea A., Rehli,M., Kabingu,E., Boch,J.A., Bare,O., Auron,P.E., Stevenson,M.A. and Calderwood,S.K. (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem., 277, 15028–15034. - PubMed
- Baker-LePain J.C., Reed,R.C. and Nicchitta,C.V. (2003) ISO: a critical evaluation of the role of peptides in heat shock/chaperone protein-mediated tumor rejection. Curr. Opin. Immunol., 15, 89–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK053058/DK/NIDDK NIH HHS/United States
- 1F32CA9016901/CA/NCI NIH HHS/United States
- HL-24066/HL/NHLBI NIH HHS/United States
- DK53058/DK/NIDDK NIH HHS/United States
- HL-65708/HL/NHLBI NIH HHS/United States
- R01 HL065708/HL/NHLBI NIH HHS/United States
- R01 HL024066/HL/NHLBI NIH HHS/United States
- R37 HL024066/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous