Leukocyte, rather than tumor-produced SPARC, determines stroma and collagen type IV deposition in mammary carcinoma - PubMed (original) (raw)
Leukocyte, rather than tumor-produced SPARC, determines stroma and collagen type IV deposition in mammary carcinoma
Sabina Sangaletti et al. J Exp Med. 2003.
Abstract
Secreted protein, acidic and rich in cysteine (SPARC), also known as osteonectin or BM-40, is a Ca2+-binding matricellular glycoprotein involved in development, wound healing, and neoplasia. However, the role of SPARC in tumors is ill defined mostly because it is expressed by both tumor and stromal cells, especially inflammatory cells. We analyzed the respective roles of host- and tumor-derived SPARC in wild-type and congenic SPARC knockout (SPARC-/-) mice on a BALB/c genetic background injected into the mammary fat pad with SPARC-producing mammary carcinoma cells derived from c-erB2 transgenic BALB/c mice. Reduced tumor growth but massive parenchyma infiltration, with large areas of necrosis and impaired vascularization were observed in SPARC-/- mice. Immunohistochemical analysis showed a defect in collagen type IV deposition in the stroma of lobular tumors from SPARC-/- mice. Chimeric mice expressing SPARC only in bone marrow-derived cells were able to organize peritumoral and perilobular stroma, whereas reciprocal chimeras transplanted with bone marrow from SPARC-/- mice developed tumors with less defined lobular structures, lacking assembled collagen type IV and with a parenchyma heavily infiltrated by leukocytes. Together, the data indicate that SPARC produced by host leukocytes, rather than the tumor, determines the assembly and function of tumor-associated stroma through the organization of collagen type IV.
Figures
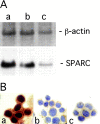
Figure 1.
SPARC expression in primary mammary carcinoma cell lines. Northern blotting (A) and immunohistochemical (B) analysis of N2C (a), N1G (b), and N3D (c) cell lines. Northern blot analysis was performed by sequential hybridization with SPARC and β-actin probes. SPARC staining localized in the cytoplasm of all three cell lines, but the higher amount of SPARC produced by N2C appeared to show an apparent staining of the nuclei, which are normally negative.
Figure 2.
Histological features and SPARC expression of N2C tumors in SPARC+/+ and SPARC−/− mice. (A) N2C tumors grew in SPARC+/+ mice as solid nests forming lobules (LB) embedded in dense, well-vascularized, connective tissue (arrowheads) and surrounded by a well-defined connective capsule (CA). Tumors from SPARC−/− mice were smaller and histologically characterized by less defined lobules, not completely surrounded by the stromal septa (arrowheads) and frequently presenting necrotic (n) areas. Original magnification, 40. (B) N2C tumor cells grown in SPARC+/+ or SPARC−/− mice produced SPARC as detected immunohistochemically with anti-SPARC mAbs. In the SPARC+/+ host, all of the cells (tumor [Tu] and stromal [S]) were strongly reactive. In the SPARC−/− host, only the tumor cells (Tu) were positive for SPARC expression, whereas the massive leukocyte infiltrate (L) invading one septum, as well as stromal cells (S) in the thin connective septa, were completely negative. Formalin fixed, paraffin embedded sections are shown. Original magnification, 200.
Figure 3.
N2C tumors in SPARC−/− mice show a defect in collagen deposition. Representative serial sections of N2C tumors grown s.c. in SPARC+/+ and SPARC−/− mice and stained with Masson's trichrome (top) and immunostained for collagen type IV (middle, Coll IV) or collagen type I (bottom, Coll I). Masson's trichrome revealed decreased collagen in the stroma of tumors from SPARC−/− mice, both at the periphery (CA, capsular area in SPARC+/+ mice) and in the connective tissue septa (arrows). Collagen type IV staining defined basement membrane structures localizing at the level of vessels and connective septa (arrows) in the wild-type mice. Collagen type IV also accounted for most of the collagen in the capsular area, where collagen type I staining was less intense. In tumors from SPARC−/− mice, the poor collagen type IV deposition did not serve to define septa or the peripheral stroma; only the basement membrane of a preexisting vessel outside the tumor area showed staining for collagen type IV (arrowhead).
Figure 4.
Stroma deposition in tumors from chimeric mice. SPARC+/+ (CB6F1, H-2bd) > SPARC−/− (BALB/c, H-2d) chimeric mice (A) and reciprocal chimeras SPARC−/− (BALB/c, H-2d) > SPARC+/+ (CB6F1, H-2b-d) mice (B) were injected s.c. with N2C tumor cells 8 wk after BMT. BMT design for each type of chimera and expression of donor MHC class I antigens in PBMCs of transplanted mice before and after BMT are shown above each panel. Cryostat sections of tumors were stained for MHC-I donor antigen, macrophages (F4/80), SPARC, and collagen type IV. In SPARC+/+ > SPARC−/− chimeras, donor (H-2Kb) SPARC-producing macrophages (F4/80+) represent the major cellular component of the stroma (S) and colocalize with a bright staining of collagen type IV. However, in tumors from SPARC−/− > SPARC+/+ chimeras, macrophages recruited from donor BM are unable to produce SPARC and are associated with reduced collagen type IV. In these tumors, necrotic (n) areas are as frequent as in tumors from SPARC−/− mice.
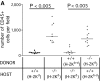
Figure 5.
Tumorigenicity of N2C cells is inversely correlated with the number of infiltrating leukocytes. Cryostat sections of tumor from SPARC+/+, SPARC−/−, SPARC+/+ > SPARC−/−, and SPARC−/− > SPARC+/+ chimeric mice were immunostained for the common leukocyte antigen CD45. Quantitative analysis of CD45+ cells (A) revealed significantly (Student's t test) higher number of CD45+ cells in tumors from SPARC−/− and SPARC−/− > SPARC+/+ chimeric mice (−/− > +/+) as compared with tumors from SPARC+/+ mice and SPARC+/+ > SPARC−/− chimeras (+/+ > −/−). (B) Localization of CD45+ cells within the tumors. In wild-type and chimeric SPARC+/+ > SPARC−/− mice, CD45+ cells were localized mainly in the connective septa completely defining lobular structures, with only a few cells infiltrating the tumor parenchyma. In tumors from SPARC−/− mice and SPARC−/− > SPARC+/+ chimeras, CD45+ cells were distributed in the residual septa, which were sometimes completely infiltrated by these cells (arrowheads) but also localized within the tumor parenchyma. Analysis of N2C tumor growth kinetics (C) showed that growth was impaired in SPARC−/− and SPARC−/− > SPARC+/+ chimeras as compared with wild-type and SPARC+/+ > SPARC−/− mice (*, P < 0.005; Student's t test at day 27). Original magnification, 100.
Figure 5.
Tumorigenicity of N2C cells is inversely correlated with the number of infiltrating leukocytes. Cryostat sections of tumor from SPARC+/+, SPARC−/−, SPARC+/+ > SPARC−/−, and SPARC−/− > SPARC+/+ chimeric mice were immunostained for the common leukocyte antigen CD45. Quantitative analysis of CD45+ cells (A) revealed significantly (Student's t test) higher number of CD45+ cells in tumors from SPARC−/− and SPARC−/− > SPARC+/+ chimeric mice (−/− > +/+) as compared with tumors from SPARC+/+ mice and SPARC+/+ > SPARC−/− chimeras (+/+ > −/−). (B) Localization of CD45+ cells within the tumors. In wild-type and chimeric SPARC+/+ > SPARC−/− mice, CD45+ cells were localized mainly in the connective septa completely defining lobular structures, with only a few cells infiltrating the tumor parenchyma. In tumors from SPARC−/− mice and SPARC−/− > SPARC+/+ chimeras, CD45+ cells were distributed in the residual septa, which were sometimes completely infiltrated by these cells (arrowheads) but also localized within the tumor parenchyma. Analysis of N2C tumor growth kinetics (C) showed that growth was impaired in SPARC−/− and SPARC−/− > SPARC+/+ chimeras as compared with wild-type and SPARC+/+ > SPARC−/− mice (*, P < 0.005; Student's t test at day 27). Original magnification, 100.
Figure 5.
Tumorigenicity of N2C cells is inversely correlated with the number of infiltrating leukocytes. Cryostat sections of tumor from SPARC+/+, SPARC−/−, SPARC+/+ > SPARC−/−, and SPARC−/− > SPARC+/+ chimeric mice were immunostained for the common leukocyte antigen CD45. Quantitative analysis of CD45+ cells (A) revealed significantly (Student's t test) higher number of CD45+ cells in tumors from SPARC−/− and SPARC−/− > SPARC+/+ chimeric mice (−/− > +/+) as compared with tumors from SPARC+/+ mice and SPARC+/+ > SPARC−/− chimeras (+/+ > −/−). (B) Localization of CD45+ cells within the tumors. In wild-type and chimeric SPARC+/+ > SPARC−/− mice, CD45+ cells were localized mainly in the connective septa completely defining lobular structures, with only a few cells infiltrating the tumor parenchyma. In tumors from SPARC−/− mice and SPARC−/− > SPARC+/+ chimeras, CD45+ cells were distributed in the residual septa, which were sometimes completely infiltrated by these cells (arrowheads) but also localized within the tumor parenchyma. Analysis of N2C tumor growth kinetics (C) showed that growth was impaired in SPARC−/− and SPARC−/− > SPARC+/+ chimeras as compared with wild-type and SPARC+/+ > SPARC−/− mice (*, P < 0.005; Student's t test at day 27). Original magnification, 100.
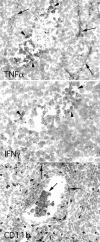
Figure 6.
Expression of TNFα and IFNγ in tumors N2C from SPARC−/− mice. In situ hybridization analysis revealed expression of TNFα mRNA both in stromal cells (arrows) and cells localized within a necrotic area (arrowheads), whereas IFNγ was detected only in cells within the necrotic area. Cells localizing in the necrotic area stained with mAb to CD11b, which defines granulocytes and macrophages.
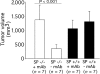
Figure 7.
Enhanced tumor growth in SPARC−/− mice treated with neutralizing antibodies against TNFα and IFNγ. Mean ± SD final volume of N2C tumors was compared 22 d after s.c. injection into SPARC+/+ and SPARC−/− mice treated i.p. with 200 μg of mAb against both TNFα and IFNγ starting 1 d after tumor cell injection. Results are from one experiment of three performed. P-value was determined by Student's t test.
Figure 8.
Immunohistochemical characterization of blood vessels associated with N2C tumors from SPARC+/+ and SPARC−/− mice. N2C tumor obtained from SPARC+/+ and SPARC−/− mice were immunostained with mAbs against CD31/PECAM-1 to characterize tumor-associated blood vessels. Although tumors from SPARC+/+ mice were highly vascularized, those from SPARC−/− mice showed a dramatic reduction in number and size of tumor-associated blood vessels both at the periphery (top; original magnification, 100) and inside the tumor (bottom; original magnification, 200).
Similar articles
- The predicted collagen-binding domains of Drosophila SPARC are essential for survival and for collagen IV distribution and assembly into basement membranes.
Duncan S, Delage S, Chioran A, Sirbu O, Brown TJ, Ringuette MJ. Duncan S, et al. Dev Biol. 2020 May 15;461(2):197-209. doi: 10.1016/j.ydbio.2020.02.011. Epub 2020 Feb 20. Dev Biol. 2020. PMID: 32087195 - SPARC oppositely regulates inflammation and fibrosis in bleomycin-induced lung damage.
Sangaletti S, Tripodo C, Cappetti B, Casalini P, Chiodoni C, Piconese S, Santangelo A, Parenza M, Arioli I, Miotti S, Colombo MP. Sangaletti S, et al. Am J Pathol. 2011 Dec;179(6):3000-10. doi: 10.1016/j.ajpath.2011.08.027. Epub 2011 Oct 11. Am J Pathol. 2011. PMID: 22001347 Free PMC article. - SPARC endogenous level, rather than fibroblast-produced SPARC or stroma reorganization induced by SPARC, is responsible for melanoma cell growth.
Prada F, Benedetti LG, Bravo AI, Alvarez MJ, Carbone C, Podhajcer OL. Prada F, et al. J Invest Dermatol. 2007 Nov;127(11):2618-28. doi: 10.1038/sj.jid.5700962. Epub 2007 Jul 12. J Invest Dermatol. 2007. PMID: 17625595 - The role of secreted protein acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis: Does expression of SPARC by macrophages influence outcomes?
Bradshaw AD. Bradshaw AD. J Mol Cell Cardiol. 2016 Apr;93:156-61. doi: 10.1016/j.yjmcc.2015.11.014. Epub 2015 Nov 12. J Mol Cell Cardiol. 2016. PMID: 26582465 Review. - Collagen IV trafficking: The inside-out and beyond story.
Chioran A, Duncan S, Catalano A, Brown TJ, Ringuette MJ. Chioran A, et al. Dev Biol. 2017 Nov 15;431(2):124-133. doi: 10.1016/j.ydbio.2017.09.037. Epub 2017 Oct 2. Dev Biol. 2017. PMID: 28982537 Review.
Cited by
- TE-array--a high throughput tool to study transposon transcription.
Gnanakkan VP, Jaffe AE, Dai L, Fu J, Wheelan SJ, Levitsky HI, Boeke JD, Burns KH. Gnanakkan VP, et al. BMC Genomics. 2013 Dec 10;14:869. doi: 10.1186/1471-2164-14-869. BMC Genomics. 2013. PMID: 24325565 Free PMC article. - The role of the extracellular matrix in primary myelofibrosis.
Leiva O, Ng SK, Chitalia S, Balduini A, Matsuura S, Ravid K. Leiva O, et al. Blood Cancer J. 2017 Feb 3;7(2):e525. doi: 10.1038/bcj.2017.6. Blood Cancer J. 2017. PMID: 28157219 Free PMC article. Review. - Microenvironmental modulation of the developing tumour: an immune-stromal dialogue.
Jones JO, Moody WM, Shields JD. Jones JO, et al. Mol Oncol. 2021 Oct;15(10):2600-2633. doi: 10.1002/1878-0261.12773. Epub 2020 Aug 28. Mol Oncol. 2021. PMID: 32741067 Free PMC article. Review. - Regulation of the fibrosis and angiogenesis promoter SPARC/osteonectin in human adipose tissue by weight change, leptin, insulin, and glucose.
Kos K, Wong S, Tan B, Gummesson A, Jernas M, Franck N, Kerrigan D, Nystrom FH, Carlsson LM, Randeva HS, Pinkney JH, Wilding JP. Kos K, et al. Diabetes. 2009 Aug;58(8):1780-8. doi: 10.2337/db09-0211. Epub 2009 Jun 9. Diabetes. 2009. PMID: 19509023 Free PMC article. - Common extracellular matrix regulation of myeloid cell activity in the bone marrow and tumor microenvironments.
Sangaletti S, Chiodoni C, Tripodo C, Colombo MP. Sangaletti S, et al. Cancer Immunol Immunother. 2017 Aug;66(8):1059-1067. doi: 10.1007/s00262-017-2014-y. Epub 2017 May 13. Cancer Immunol Immunother. 2017. PMID: 28501940 Free PMC article. Review.
References
- Balkwill, F., and A. Mantovani. 2001. Inflammation and cancer: back to Virchow? Lancet. 17:539–544. - PubMed
- Moore, R., D. Owens, G. Stamp, C. Arnott, F. Burke, N. East, H. Holdsworth, L. Turner, B. Rollins, M. Pasparakis, et al. 1999. Tumour necrosis factor-α deficient mice are resistant to skin carcinogenesis. Nat. Med. 5:828–831. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous