Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0 - PubMed (original) (raw)
Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0
Alice P W Poon et al. J Virol. 2003 Dec.
Abstract
An earlier report showed that the expression of viral genes by a herpes simplex virus 1 mutant [HSV-1(vCPc0)] in which the wild-type, spliced gene encoding infected-cell protein no. 0 (ICP0) was replaced by a cDNA copy is dependent on both the cell type and multiplicity of infection. At low multiplicities of infection, viral gene expression in rabbit skin cells was delayed by many hours, although ultimately virus yield was comparable to that of the wild-type virus. This defect was rescued by replacement of the cDNA copy with the wild-type gene. To test the hypothesis that the delay reflected a dysfunction of ICP0 in altering the structure of host protein-viral DNA complexes, we examined the state of histone deacetylases (HDACs) (HDAC1, HDAC2, and HDAC3). We report the following. (i) HDAC1 and HDAC2, but not HDAC3, were modified in infected cells. The modification was mediated by the viral protein kinase U(S)3 and occurred between 3 and 6 h after infection with wild-type virus but was delayed in rabbit skin cells infected with HSV-1(vCPc0) mutant, concordant with a delay in the expression of viral genes. (ii) Pretreatment of rabbit skin cells with inhibitors of HDAC activity (e.g., sodium butyrate, Helminthosporium carbonum toxin, or trichostatin A) accelerated the expression of HSV-1(vCPc0) but not that of wild-type virus. We conclude the following. (i) In the interval in which HSV-1(vCPc0) DNA is silent, its DNA is in chromatin-like structures amenable to modification by inhibitors of histone deacetylases. (ii) Expression of wild-type virus genes in these cells precluded the formation of DNA-protein structures that would be affected by either the HDACs or their inhibitors. (iii) Since the defect in HSV-1(vCPc0) maps to ICP0, the results suggest that this protein initiates the process of divestiture of viral DNA from tight chromatin structures but could be replaced by other viral proteins in cells infected with a large number of virions.
Figures
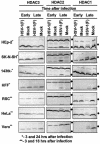
FIG. 1.
Electrophoretic profiles of HDAC1, HDAC2, and HDAC3 in wild-type HSV-1(F) virus-infected cells at early and late times after infection. Replicate cultures of HEp2, SK-N-SH, 143TK−, HFF, RSC, HeLa, or Vero cells in 25-cm2 flasks were either mock infected or infected with 5 PFU of HSV-1(F) per cell. The cells were harvested at early (3 h) or late (18 h [**] or 24 h [*]) times after infection. Proteins were solubilized in disruption buffer and electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, and reacted with polyclonal antibodies to HDAC1, HDAC2, and HDAC3 as described in Materials and Methods. The positions of posttranslationally modified protein bands are indicated by the solid circles to the right of the blots.
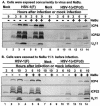
FIG. 2.
Electrophoretic profiles of HDAC1 and HDAC2 in RSC infected with wild-type and mutant viruses at early and late times after infection. Replicate cultures of RSC in 25-cm2 flasks were either mock infected (lane 7) or infected with wild-type HSV-1(F), HSV-1 (17), HSV-1(KOS), or HSV-2(G), mutant HSV-1(vCPc0), or repaired HSV-1(vCPc0)R. Cell cultures were infected with 10 PFU of virus per cell. Cells were harvested at 6.5 h (lanes 1 to 6) or 21 h (lanes 7 to 13) after infection. Proteins were solubilized in disruption buffer and electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, and reacted with polyclonal antibodies to HDAC1 and HDAC2 as described in Materials and Methods. The positions of HDAC1 and HDAC2 and of bands reacting with HDAC2 antibody (V) are indicated to the right of the gel.
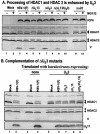
FIG. 3.
Modification of HDAC1 and HDAC2. (A) Processing of HDAC1 and HDAC2 is enhanced by viral protein kinase US3. Replicate cultures of RSC in 25-cm2 flasks were either mock infected or infected with wild-type HSV-1(F) or mutant R7356 (ΔUL13), R7041 (ΔUS3), R7353 (ΔUL13/ΔUS3), or R7802 (Δα22). Cell cultures were infected with 5 PFU of virus per cell. At 3 h after infection, one set of cultures was replenished with fresh untreated medium (− lanes), whereas a second set was replenished with medium containing 10 μM MG132 (+ lanes). The cells were harvested 20 h after infection. Proteins were solubilized in disruption buffer, electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, and reacted with polyclonal antibodies to HDAC1 and HDAC2, and monoclonal antibody to ICP0. The positions of HDAC1 and HDAC2 and of bands reacting with HDAC2 antibody (V) and with monoclonal antibody to ICP0 are indicated to the right of the gel. (B) Complementation of ΔUS3 mutants by baculovirus expressing US3. Replicate RSC cultures in 25-cm2 flasks were transduced with baculoviruses that were either empty of inserts (lanes 3 and 4) or encoding the US3 protein kinase (lanes 5 to 9). At 12 h after transduction, cells were mock infected (lanes 3 and 5) or infected with wild-type HSV-1(F) (lanes 4 and 6) or mutant virus R7356 (ΔUL13), R7041 (ΔUS3), or R7353 (ΔUL13/ΔUS3) (lanes 7 to 9). Cell cultures were infected with 5 PFU of virus per cell. At 12 h after infection with wild-type HSV-1 or mutant viruses, the cultures were harvested and processed as described above except that cell lysates were reacted with anti-US11 antibody rather than ICP0. Lanes 1 and 2 show protein profiles of mock-infected and wild-type virus-infected cell cultures not previously exposed to baculoviruses.
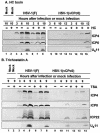
FIG. 4.
Temporal pattern of accumulation of selected wild-type HSV-1(F) and mutant HSV-1(vCPc0) proteins in RSC treated with sodium butyrate (NaBu) (A) at the time of infection and (B) 11 h before infection. (A) Replicate cultures of RSC in 25-cm2 flasks were either mock infected (lanes 1 and 8) or infected with 5 PFU of wild-type HSV-1(F) per cell (lanes 2 to 7) or with 5 PFU of mutant HSV-1(vCPc0) per cell (lanes 9 to 14) in the absence (−) or presence (+) of 6 mM sodium butyrate. The cells were harvested at the indicated times after infection. Proteins were solubilized in disruption buffer, electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, and reacted with polyclonal antibody to ICP22 and monoclonal antibodies to ICP4 and US11. (B) Replicate cultures of RSC in 25-cm2 flasks were either mock treated (lanes 1 to 3, 7, and 9 to 11) or treated (lanes 4 to 6, 8, and 12 to 14) with 6 mM sodium butyrate. At 11 h after treatment, cells were either mock infected (lanes 7 and 8) or infected with 5 PFU of wild-type HSV-1(F) per cell (lanes 1 to 6) or with 5 PFU of mutant HSV-1(vCPc0) per cell (lanes 9 to 14) in the absence (−) or presence (+) of 6 mM sodium butyrate. Cells were harvested at the indicated times after infection. Proteins were solubilized in disruption buffer, electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, and reacted with polyclonal antibody to ICP22 and monoclonal antibodies to ICP4 and US11.
FIG. 5.
Temporal pattern of accumulation of selected wild-type HSV-1(F) and mutant HSV-1(vCPc0) proteins in RSC treated with H. carbonum (HC) toxin (A) and trichostatin A (B) 11 h before infection. (A) Replicate cultures of RSC in 25-cm2 flasks were either mock treated (−) or treated (+) with 70 ng of H. carbonum toxin per ml. At 11 h after treatment, cells were either mock infected (lanes 1 and 2) or infected with 5 PFU of wild-type HSV-1(F) per cell (lanes 3 to 8) or with 5 PFU of mutant HSV-1(vCPc0) per cell (lanes 9 to 16) in the absence (−) or presence (+) of 70 ng of H. carbonum toxin per ml. Cells were harvested at the indicated times after infection. Proteins were solubilized in disruption buffer, electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, and reacted with polyclonal antibody to ICP22 and monoclonal antibodies to ICP0, ICP4, and US11. (B) The experiment was repeated with 150 ng of trichostatin A (TSA) per ml, instead of H. carbonum toxin.
Similar articles
- ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression.
Poon AP, Gu H, Roizman B. Poon AP, et al. Proc Natl Acad Sci U S A. 2006 Jun 27;103(26):9993-8. doi: 10.1073/pnas.0604142103. Epub 2006 Jun 19. Proc Natl Acad Sci U S A. 2006. PMID: 16785443 Free PMC article. - Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex.
Gu H, Roizman B. Gu H, et al. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):17134-9. doi: 10.1073/pnas.0707266104. Epub 2007 Oct 15. Proc Natl Acad Sci U S A. 2007. PMID: 17939992 Free PMC article. - The checkpoints of viral gene expression in productive and latent infection: the role of the HDAC/CoREST/LSD1/REST repressor complex.
Roizman B. Roizman B. J Virol. 2011 Aug;85(15):7474-82. doi: 10.1128/JVI.00180-11. Epub 2011 Mar 30. J Virol. 2011. PMID: 21450817 Free PMC article. Review. - The first 30 minutes in the life of a virus: unREST in the nucleus.
Roizman B, Gu H, Mandel G. Roizman B, et al. Cell Cycle. 2005 Aug;4(8):1019-21. doi: 10.4161/cc.4.8.1902. Epub 2005 Aug 7. Cell Cycle. 2005. PMID: 16082207 Review.
Cited by
- Human cytomegalovirus pUL97 regulates the viral major immediate early promoter by phosphorylation-mediated disruption of histone deacetylase 1 binding.
Bigley TM, Reitsma JM, Mirza SP, Terhune SS. Bigley TM, et al. J Virol. 2013 Jul;87(13):7393-408. doi: 10.1128/JVI.02825-12. Epub 2013 Apr 24. J Virol. 2013. PMID: 23616659 Free PMC article. - Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle.
Perng GC, Jones C. Perng GC, et al. Interdiscip Perspect Infect Dis. 2010;2010:262415. doi: 10.1155/2010/262415. Epub 2010 Feb 15. Interdiscip Perspect Infect Dis. 2010. PMID: 20169002 Free PMC article. - Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo.
Thompson RL, Sawtell NM. Thompson RL, et al. J Virol. 2006 Nov;80(22):10919-30. doi: 10.1128/JVI.01253-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943285 Free PMC article. - Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1.
Hagglund R, Roizman B. Hagglund R, et al. J Virol. 2004 Mar;78(5):2169-78. doi: 10.1128/jvi.78.5.2169-2178.2004. J Virol. 2004. PMID: 14963113 Free PMC article. Review. No abstract available. - Histone deacetylase inhibitors induce reactivation of herpes simplex virus type 1 in a latency-associated transcript-independent manner in neuronal cells.
Danaher RJ, Jacob RJ, Steiner MR, Allen WR, Hill JM, Miller CS. Danaher RJ, et al. J Neurovirol. 2005 Jul;11(3):306-17. doi: 10.1080/13550280590952817. J Neurovirol. 2005. PMID: 16036811 Free PMC article.
References
- Chelbi-Alix, M. K., and H. de The. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear body-associated PML and Sp100 proteins. Oncogene 18:935-941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA78766/CA/NCI NIH HHS/United States
- CA83939/CA/NCI NIH HHS/United States
- R01 CA078766/CA/NCI NIH HHS/United States
- CA71933/CA/NCI NIH HHS/United States
- CA87661/CA/NCI NIH HHS/United States
- P01 CA087661/CA/NCI NIH HHS/United States
- P01 CA071933/CA/NCI NIH HHS/United States
- R01 CA088860/CA/NCI NIH HHS/United States
- CA88860/CA/NCI NIH HHS/United States
- R37 CA078766/CA/NCI NIH HHS/United States
- R01 CA083939/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous