The safety of over-the-counter niacin. A randomized placebo-controlled trial [ISRCTN18054903] - PubMed (original) (raw)
Clinical Trial
The safety of over-the-counter niacin. A randomized placebo-controlled trial [ISRCTN18054903]
Edward Mills et al. BMC Clin Pharmacol. 2003.
Abstract
Background: Niacin is widely available over the counter (OTC). We sought to determine the safety of 500 mg immediate release niacin, when healthy individuals use them as directed.
Methods: 51 female and 17 male healthy volunteers (mean age 27 years SD 4.4) participated in a randomized placebo-controlled blinded trial of a single dose of an OTC, immediate-release niacin 500 mg (n = 33), or a single dose of placebo (n = 35) on an empty stomach. The outcomes measured were self-reported incidence of flushing and other adverse effects.
Results: 33 volunteers on niacin (100%) and 1 volunteer on placebo (3%) flushed (relative risk 35, 95% confidence interval (CI) 6.8-194.7). Mean time to flushing on niacin was 18.2 min (95% CI: 12.7-23.6); mean duration of flushing was 75.4 min (95% CI: 62.5-88.2). Other adverse effects occurred commonly in the niacin group: chills (51.5% vs. 0%, P <.0001), generalized pruritus (75% vs. 0%, P = <.001), gastrointestinal upset (30% vs. 3%, P =.005), and cutaneous tingling (30% vs. 0%, P = <.001). Six participants did not tolerate the adverse effects of niacin and 3 required medical attention.
Conclusion: Clinicians counseling patients about niacin should alert patients not only about flushing but also about gastrointestinal symptoms, the most severe in this study. They should not trust that patients would receive information about these side effects or their prevention (with aspirin) from the OTC packet insert.
Figures
Figure 1
Results form.
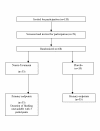
Figure 2
Flow of participants throughout the trial.
Similar articles
- Aspirin reduces cutaneous flushing after administration of an optimized extended-release niacin formulation.
Cefali EA, Simmons PD, Stanek EJ, McGovern ME, Kissling CJ. Cefali EA, et al. Int J Clin Pharmacol Ther. 2007 Feb;45(2):78-88. doi: 10.5414/cpp45078. Int J Clin Pharmacol Ther. 2007. PMID: 17323787 Clinical Trial. - Improved control of niacin-induced flushing using an optimized once-daily, extended-release niacin formulation.
Cefali EA, Simmons PD, Stanek EJ, Shamp TR. Cefali EA, et al. Int J Clin Pharmacol Ther. 2006 Dec;44(12):633-40. doi: 10.5414/cpp44633. Int J Clin Pharmacol Ther. 2006. PMID: 17190373 Clinical Trial. - Validation of a questionnaire to assess niacin-induced cutaneous flushing.
Norquist JM, Watson DJ, Yu Q, Paolini JF, McQuarrie K, Santanello NC. Norquist JM, et al. Curr Med Res Opin. 2007 Jul;23(7):1549-60. doi: 10.1185/030079907x199637. Curr Med Res Opin. 2007. PMID: 17559750 Clinical Trial. - Safety and tolerability of extended-release niacin with laropiprant.
Yadav R, Kwok S, Ammori BJ, Issa B, Soran H. Yadav R, et al. Expert Opin Drug Saf. 2012 Jan;11(1):151-9. doi: 10.1517/14740338.2011.638281. Epub 2011 Dec 1. Expert Opin Drug Saf. 2012. PMID: 22133050 Review.
Cited by
- Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target.
Behbodikhah J, Ahmed S, Elyasi A, Kasselman LJ, De Leon J, Glass AD, Reiss AB. Behbodikhah J, et al. Metabolites. 2021 Oct 8;11(10):690. doi: 10.3390/metabo11100690. Metabolites. 2021. PMID: 34677405 Free PMC article. Review. - Cost-effectiveness of extended-release niacin/laropiprant added to a stable simvastatin dose in secondary prevention patients not at cholesterol goal in Germany.
Michailov GV, Davies GM, Krobot KJ. Michailov GV, et al. Eur J Health Econ. 2012 Jun;13(3):365-74. doi: 10.1007/s10198-011-0309-z. Epub 2011 Apr 5. Eur J Health Econ. 2012. PMID: 21465286 Free PMC article. - Balancing NAD+ deficits with nicotinamide riboside: therapeutic possibilities and limitations.
Cercillieux A, Ciarlo E, Canto C. Cercillieux A, et al. Cell Mol Life Sci. 2022 Aug 2;79(8):463. doi: 10.1007/s00018-022-04499-5. Cell Mol Life Sci. 2022. PMID: 35918544 Free PMC article. Review. - Extended-release niacin/laropiprant significantly improves lipid levels in type 2 diabetes mellitus irrespective of baseline glycemic control.
Bays HE, Brinton EA, Triscari J, Chen E, Maccubbin D, MacLean AA, Gibson KL, Ruck RA, Johnson-Levonas AO, O'Neill EA, Mitchel YB. Bays HE, et al. Vasc Health Risk Manag. 2015 Feb 24;11:165-72. doi: 10.2147/VHRM.S70907. eCollection 2015. Vasc Health Risk Manag. 2015. PMID: 25750540 Free PMC article. Clinical Trial. - Nicotinamide riboside is uniquely and orally bioavailable in mice and humans.
Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Trammell SA, et al. Nat Commun. 2016 Oct 10;7:12948. doi: 10.1038/ncomms12948. Nat Commun. 2016. PMID: 27721479 Free PMC article.
References
- FDA Center for Food Safety & Applied Nutrition Dietary Supplements. http://vm.cfsan.fda.gov/~dms/supplmnt.html Dec.10, 2002.
- HealthCanada. Dept of Justice. Food and Drugs Act. ( RS 1985, c F-27 )
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical