A class-V myosin required for mating, hyphal growth, and pathogenicity in the dimorphic plant pathogen Ustilago maydis - PubMed (original) (raw)
. 2003 Dec;15(12):2826-42.
doi: 10.1105/tpc.016246. Epub 2003 Nov 13.
Affiliations
- PMID: 14615599
- PMCID: PMC282809
- DOI: 10.1105/tpc.016246
A class-V myosin required for mating, hyphal growth, and pathogenicity in the dimorphic plant pathogen Ustilago maydis
Isabella Weber et al. Plant Cell. 2003 Dec.
Abstract
In the early stages of plant infection, yeast-like haploid sporidia of Ustilago maydis respond to pheromone secreted by compatible partners by forming conjugation tubes. These then fuse to generate a dikaryotic hypha that forms appressoria to penetrate the host plant. As a first step toward understanding the structural requirements for these transitions, we have identified myo5, which encodes a class-V myosin. Analysis of conditional and null mutants revealed that Myo5 plays nonessential roles in cytokinesis and morphogenesis in sporidia and is required for hyphal morphology. Consistent with a role in morphogenesis, a functional green fluorescent protein-Myo5 fusion protein localized to the bud tip and the hyphal apex as well as to the septa and the spore wall during later stages of infection. However, the loss of Myo5 did not affect the tip growth of hyphae and sporidia. By contrast, Myo5 was indispensable for conjugation tube formation. Furthermore, myo5 mutants were impaired in the perception of pheromones, which indicates a particular importance of Myo5 in the mating process. Consequently, few mutant hyphae were formed that penetrated the plant epidermis but did not continue invasive growth. These results indicate a crucial role of Myo5 in the morphogenesis, dimorphic switch, and pathogenicity of U. maydis.
Figures
Figure 1.
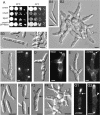
Myo5 Is a Class-V Myosin. (A) A dendrogram of myosins created by the distance-based minimum-evolution method, based on 500 replicates. Note that U. maydis contains one myosin of class V, I, and II. Bootstrap values are given, and branching points and the scale bar denote substitutions per site. Sc, Saccharomyces cerevisiae; Um, Ustilago maydis; Sp, Schizosaccharomyces pombe; Mm, Mus musculus; Gg, Gallus gallus; Dd, Dictyostelium discoideum; An, Aspergillus nidulans. (B) Dot plot comparison of Myo5 and Myo2p from S. cerevisiae. Note that both motors share significant sequence identity over the entire length. (C) Comparison of the domain structures of Myo5 and Myo2p from S. cerevisiae, Myo52/Myo4 of S. pombe, and mouse Myo5A. Myo5 contains all predicted domains that are typical for class-V myosins. AA, amino acids.
Figure 2.
Characterization of Δ_myo5_ and Temperature-Sensitive myo5_ts Mutants. (A) Plate growth of FB2 (control) and myosin mutants at 20 and 34°C. At low temperature, the growth of control cells, Δ_myo5, and myo5_ts is indistinguishable. However, both Δ_myo5 and myo5_ts show growth defects at 34°C that can be rescued by the expression of a functional GFP-Myo5 fusion protein. Note that myo5 is not essential for the survival of U. maydis. (B) Morphology defect of Δ_myo5 cells. Wild-type cells are cigar shaped and form polar buds ([B1]). By contrast, deletion of myo5 leads to very thick and irregularly shaped cell aggregates. Most cells do not separate but form septa (arrow in [B2]). Interestingly, most Δ_myo5_ cells are elongated (arrowhead in [B3]), whereas older cells often lose polarity and are rounded (arrow in [B3]). Bars = 5 μm. (C) Polar distribution of F-actin patches in Δ_myo5_ stained with anti-actin antibodies. Bar = 5 μm. (D) Chitin distribution in Δ_myo5_. Rhodamine-labeled wheat germ agglutinin (WGA) detects newly synthesized chitin at cell tips (arrow), indicating that Δ_myo5_ mutants grow in a polar manner. Bar = 5 μm. (E) Localization of chitin synthase detected with a cross-reactive antibody raised against Chs2p from S. cerevisiae (Sietsma et al., 1996) in Δ_myo5_ mutants. The antibody most likely detects chitin synthases at the growing tip of Δ_myo5_ cells, in agreement with the WGA staining of chitin. Bar = 5 μm. (F) Morphology of temperature-sensitive _myo5_ts mutants. At the permissive temperature (20°C, [F1]), FB2Myo5ts strains grow by polar budding but are significantly thicker than control cells (see [B1] for comparison). At the restrictive temperature (34°C), mutant cells show a defect in dissolving the cell wall after cytokinesis (arrow in [F2]; 1.5 h at 34°C). At later stages at 34°C, they form septa (arrow in [F3]; 6 h at 34°C) and start branching. After extended growth at 34°C, myo5_ts mutants form large aggregates that are reminiscent of Δ_myo5 cells. Bars = 5 μm in (F1) to (F3) and 10 μm in (F4). (G) Chitin synthase distribution in _myo5_ts cells. At the permissive temperature, anti-Chs2p antibodies detect punctate staining in the growing bud, a ring at the bud neck (arrow in [G1]), and the distal cell pole. At 34°C, this chitin synthase distribution is largely unaffected, although more anti-Chs2p signals are detected at the periphery (arrowhead in [G2]). Note that the distribution of anti-Chs2p signals is very much in agreement with chitin staining (not shown) and the expected localization of chitin syntheses at the growth region, suggesting that the cross-reactive antibody is specific for chitin synthase in U. maydis. Bar = 5 μm.
Figure 3.
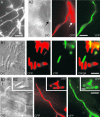
Localization of a Functional GFP-Myo5 Fusion Protein in Haploid Sporidia. (A) GFP-Myo5 distribution during stages of the cell cycle. GFP-Myo5, expressed under the control of its native promoter, rescues the phenotype of Δ_myo5_ cells. In presumably nongrowing cells, GFP-Myo5 shows a dispersed localization at the cell periphery (A1). Before bud formation, cells begin to polarize and GFP-Myo5 concentrates at one cell pole (A2). In small-budded cells, GFP-Myo5 remains at the bud tip (A3), whereas in cells with medium-sized buds, the fusion protein localizes to the growing and the nongrowing ends of the cell (arrow in [A4]). Finally, it accumulates at the site of cell cleavage ([A5]; inset shows the equal signal intensity on both sides of the formed septa). Note that the distal localization of GFP-Myo5 (arrow in [A4]) coincides with the appearance of endosomes at this cell pole (Wedlich-Söldner et al., 2000, 2002), which suggests exocytotic activity at this cell end. Bar = 5 μm. (B) Distribution of GFP-Myo5 and immunolabeled actin patches during the cell cycle of haploid U. maydis. During the early stages of budding, GFP-Myo5 (green) and actin (red) colocalize ([B1] to [B3]; overlay results in yellow). However, in large budded cells, actin appears at the bud neck (arrows in [B4] and [B5]), whereas most GFP-Myo5 still localizes to the growing bud tip. During cytokinesis, both GFP-Myo5 and actin concentrate at the cleavage site (B6). Bar = 5 μm. (C) Upon oxygen depletion caused by prolonged observation, GFP-Myo5 localizes to filamentous structures. The inset shows a large bud that contains filaments that are in contact with the GFP-Myo5 accumulation at the tip. Bar = 5 μm. (D) GFP-Myo5 localization after ATP depletion. In untreated cells expressing high levels of GFP-Myo5 (FB2oGMyo5), the tip signal intensity increases (D1). After treatment with CCCP, which inhibits the mitochondrial respiration chain and therefore depletes ATP, GFP-Myo5 stains long filaments (D2). These structures can be disrupted by the actin inhibitor cytochalasin D (CD [D3]), suggesting that they are F-actin cables. Bars = 5 μm. (E) Localization of GFP-Myo5 after the disruption of microtubules, F-actin, or inhibited myosin activity. Treatment with the solvent DMSO (not shown) or benomyl (Ben [E1]) at concentrations that were shown to disrupt all microtubules (20 μM) (Straube et al., 2003) does not significantly affect GFP-Myo5 distribution. However, 50 μM of the potent F-actin inhibitor latrunculin A (LatA [E2]) as well as 10 mM of the myosin inhibitor BDM (E3) abolish the polar accumulation of GFP-Myo5, suggesting that actin-dependent transport underlies the tip localization of Myo5. Bar = 5 μm.
Figure 4.
Localization of the GFP-Myo5 Fusion Protein in Hyphae. (A) GFP-Myo5 localizes to the tip of dikaryotic hyphae derived from crossing FB2mGM and FB1 on charcoal plates. Note that no signal is detected at the septum (arrows) and that the tip staining varies from pointed dots to dispersed gradients (not shown). Bar = 10 μm. (B) GFP-Myo5 in monokaryotic hyphae from liquid culture. Because of genetic manipulation, filamentous growth of strain AB33oGM can be induced by shifting cells to liquid medium containing nitrate. In these cells, GFP-Myo5 is restricted to a bright dot at the very tip of the hyphae. Note that prolonged observation results in dispersed distribution and filament staining, suggesting that these localization patterns reflect suboptimal growth conditions. Bar = 5 μm. (C) Treatment with the solvent DMSO only slightly affects GFP-Myo5 distribution (C1) in AB33oGM. The addition of 20 μM benomyl (Ben) results in dispersed gradients of the fusion protein that lost the pointed localization (C2). By contrast, gradients are almost absent with 10 mM BDM (C3) and disappear completely in the presence of 50 μM latrunculin A (LatA [C4]). Bar = 5 μm. (D) Quantitative analysis of GFP-Myo5 distribution in inhibitor-treated AB33oGM hyphae. (E) Distribution of GFP-Myo5 in hyphae lacking Kin2, a conventional kinesin from U. maydis. On charcoal plates (E1), the control strain SG200 forms white colonies, whereas the kin2 deletion mutant that expresses GFP-Myo5 (Δ_kin2_oGM) rarely forms filaments, thereby appearing gray on charcoal plates (Lehmler et al., 1997). In liquid culture, these hyphae contain an apical GFP-Myo5 dot (E2), demonstrating that conventional kinesin is not involved in Myo5 transport to the hyphal apex. Bar = 5 μm.
Figure 5.
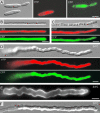
Analysis of the Formation of Dikaryotic myo5_ts Hyphae on Charcoal-Containing Plates. Wild-type strains (WT × WT, 28°C) formed white colonies that consist mainly of dikaryotic hyphae. Crossing of wild-type and Δ_myo5 strains shows drastically impaired filament formation (WT × Δmyo5, 28°C) that is restored by expression of the functional GFP-Myo5 fusion protein (WT × Δmyo5GFPMyo5). Mating reactions of wild-type and temperature-sensitive _myo5_ts strains show no defects in filament formation at permissive and restrictive temperatures (WT × Myo5ts, 20 and 28°C). The formation of dikaryotic hyphae is impaired in crosses of _myo5_ts cells under permissive conditions (Myo5ts × Myo5ts, 20°C) and is abolished completely at the restrictive temperature (Myo5ts × Myo5ts, 28°C). However, filamentation is restored by incubation at the permissive temperature for 2 days followed by growth at the restrictive temperature (Myo5ts × Myo5ts, 20 to 28°C). This finding suggests that _myo5_ts strains fail to form filaments at an early stage of the mating process but hyphae appear after the initial fusion is complete.
Figure 6.
Defects of _myo5_ts Mutants during Early Steps of Mating. (A) At the beginning of mating and before conjugation tube formation, secreted pheromone of the opposing mating partner is recognized and mfa1 expression increases. In _myo5_ts and control strains, GFP was put under the control of the mfa1 promoter, which allows the visualization of pheromone perception. In addition, the ability to form conjugation tubes was monitored in confrontation assays, in which adjacent mating partners bridge large distances by the formation of conjugation hyphae. In a negative control of cells of the same mating type, tubes are not formed and _mfa-_GFP expression is not detected (A1). In compatible confrontations of compatible strains, long conjugation tubes are formed, but only the _mfa-_GFP–containing strain shows GFP fluorescence (A2), whereas both the perception of pheromone and tube formation are detectable in confrontation assays of FB2mG and FB1mG at 20°C (A3) and 28°C (A4). By contrast, plating _mfa-_GFP–containing _myo5_ts strains at the permissive temperature does not result in tube formation, nor are cells properly stimulated (A5). The mating reaction is improved after reducing the distance between the compatible _myo5_ts strains, but only short hyphae are formed (A6). Filamentation of mutant cells is abolished at 28°C (A7). However, _myo5_ts mutants are able to stimulate compatible control cells (A8). All images were taken after 14 h of incubation. Bar in (A1) = 50 μm. (B) Treatment of strain FB2mG with synthetic _a1_-mating pheromone for 8 h at 20°C induces the expression of _mfa-_GFP and the formation of hyphal extensions that most likely resemble conjugation tubes (B1). Occasionally, cells without GFP expression are observed that also do not grow filamentously (B2). Bars = 20 μm. (C) FB2Myo5ts mG cells treated with pheromone are only partially stimulated and do not form any tubes after 8 h at 20°C (C1). After 14 h, only stimulated cells, identified by their GFP signals, form short and irregular extensions that resemble those depicted in (A6). Bars = 20 μm.
Figure 7.
Analysis of the Role of Myo5 in Dikaryotic Hyphae. (A) Expression of yellow- and cyan-shifted derivatives of GFP (YFP and CFP) allows the identification of mating partners that contain compatible mating loci. Signals are given in false colors. Bar = 5 μm. (B) Fusion of compatible haploid wild-type strains expressing either YFP or CFP results in dikaryotic hyphae that contain both fusion proteins (YFP in red; CFP in green). Bar = 5 μm. (C) Crossing CFP and YFP expressing compatible _myo5_ts strains at the permissive temperature resulted in straight but slightly thicker dikaryotic hyphae, as confirmed by the detection of both YFP (red) and CFP (green) in a single cell. Bar = 5 μm. (D) After mating at the permissive temperature and growth for another day at the restrictive temperature, dikaryotic mutant hyphae are thicker and grow irregularly. Note that this image is given at a lower magnification than that in (C). Bar = 5 μm. (E) Chitin staining in _myo5_ts hyphae under restrictive conditions. In contrast to wild-type hyphae, which accumulate chitin at the growing hyphal tip (not shown), WGA staining reveals the irregular composition of the mutant cell wall. This finding suggests that the abnormal morphology of _myo5_ts hyphae is attributable to defects in the cell wall. Bar = 5 μm. (F) At 28°C, _myo5_ts hyphae show the temperature-dependent phenotype. Bar = 5 μm.
Figure 8.
_Myo5_ts Mutants Cells on the Epidermis of the Host Plant. (A) Infections of young maize plants with a mixture of compatible wild-type cells results in a network of hyphae that can be detected on the plant surface after 2 days by Calcofluor staining of the fungal cell wall (A1). These filaments are derived from the fusion of mating partners, as confirmed by infection with CFP- and YFP-expressing compatible strains (A2). DIC, differential interference contrast. Bars = 10 μm. (B) Infection of plants at 20°C with compatible _myo5_ts strains that express YFP or CFP demonstrates that most mutant cells do not fuse with adjacent mating partners, and only rarely do two cells fuse, thereby expressing both CFP and YFP (B2). Consistent with the in vitro observation on charcoal plates, the fusion of compatible cells results in the formation of long dikaryotic infection hyphae (B3). Bars = 10 μm in (B1) and (B3) and 5 μm in (B2).
Figure 9.
Penetration of the Host Plant by Wild-Type and _myo5_ts Hyphae. (A) Series of minimal _Z_-axis projections showing a top view of the initial infection state of wild-type hypha at 20°C. One day after infection (1 dpi), wild-type hyphae form appressoria on the plant surface (arrowhead; −11.5 to 0 μm), usually invade the plant as an unbranched hypha (0 to 18 μm), and reach through the epidermis into the underlying mesophyll of the leaf (18 to 36 μm). Note that hyphae often penetrate into much deeper regions of the leaf (the arrow points to a branch that leaves the focal plane). Bar = 10 μm. (B) _myo5_ts mutant hypha 6 days after infection (6 dpi) at 20°C. An appressorium is formed (arrow; 8.7 to 0 μm), and the hypha penetrates straight through the epidermis (0 to 20 μm), but it does form multiple and swollen branches within the leaf mesophyll (40 to 60 μm and 60 to 77 μm). Note that images are top views of minimal _Z_-axis projections of planes within the indicated range. Bar = 10 μm. (C) Graph based on minimum projections of 456 planes (WT × WT) and 771 planes (Myo5ts × Myo5ts) that illustrates the dimensions and extent of branching of _myo5_ts hyphae. The gray circle indicates the site of entry into the plant. Bar = 10 μm. (D) GFP-Myo5 localization in planta. GFP-Myo5 localizes in tips of growing hyphae on the plant surface (D1). Six days after infection, hyphae differentiate inside infected plants and GFP-Myo5 concentrates at the tips of lobed branches (D2) and septa (D3). After 13 to 14 days, hyphal fragmentation occurs, cells become rounded, and GFP-Myo5 appears at the tips and septa of these cells (arrows in [D4]). Finally, spore maturation occurs and spore walls are formed. GFP-Myo5 localizes in patches at the cell periphery. DIC, differential interference contrast. Bars = 5 μm.
Similar articles
- Sustained cell polarity and virulence in the phytopathogenic fungus Ustilago maydis depends on an essential cyclin-dependent kinase from the Cdk5/Pho85 family.
Castillo-Lluva S, Alvarez-Tabarés I, Weber I, Steinberg G, Pérez-Martín J. Castillo-Lluva S, et al. J Cell Sci. 2007 May 1;120(Pt 9):1584-95. doi: 10.1242/jcs.005314. Epub 2007 Apr 3. J Cell Sci. 2007. PMID: 17405809 - An Ustilago maydis septin is required for filamentous growth in culture and for full symptom development on maize.
Boyce KJ, Chang H, D'Souza CA, Kronstad JW. Boyce KJ, et al. Eukaryot Cell. 2005 Dec;4(12):2044-56. doi: 10.1128/EC.4.12.2044-2056.2005. Eukaryot Cell. 2005. PMID: 16339722 Free PMC article. - Polar growth in the infectious hyphae of the phytopathogen ustilago maydis depends on a virulence-specific cyclin.
Flor-Parra I, Castillo-Lluva S, Pérez-Martín J. Flor-Parra I, et al. Plant Cell. 2007 Oct;19(10):3280-96. doi: 10.1105/tpc.107.052738. Epub 2007 Oct 5. Plant Cell. 2007. PMID: 17921314 Free PMC article. - [Parasitic strategy and regulation mechanism of Ustilago maydis - A review].
Li Z, Yan L, Yan Z. Li Z, et al. Wei Sheng Wu Xue Bao. 2016 Sep;56(9):1385-97. Wei Sheng Wu Xue Bao. 2016. PMID: 29738207 Review. Chinese. - Sex in smut fungi: Structure, function and evolution of mating-type complexes.
Bakkeren G, Kämper J, Schirawski J. Bakkeren G, et al. Fungal Genet Biol. 2008 Aug;45 Suppl 1:S15-21. doi: 10.1016/j.fgb.2008.04.005. Epub 2008 May 22. Fungal Genet Biol. 2008. PMID: 18501648 Review.
Cited by
- Ustilago maydis: how its biology relates to pathogenic development.
Kahmann R, Kämper J. Kahmann R, et al. New Phytol. 2004 Oct;164(1):31-42. doi: 10.1111/j.1469-8137.2004.01156.x. New Phytol. 2004. PMID: 33873482 Review. - Microtubules are dispensable for the initial pathogenic development but required for long-distance hyphal growth in the corn smut fungus Ustilago maydis.
Fuchs U, Manns I, Steinberg G. Fuchs U, et al. Mol Biol Cell. 2005 Jun;16(6):2746-58. doi: 10.1091/mbc.e05-03-0176. Epub 2005 Apr 13. Mol Biol Cell. 2005. PMID: 15829564 Free PMC article. - Polar localizing class V myosin chitin synthases are essential during early plant infection in the plant pathogenic fungus Ustilago maydis.
Weber I, Assmann D, Thines E, Steinberg G. Weber I, et al. Plant Cell. 2006 Jan;18(1):225-42. doi: 10.1105/tpc.105.037341. Epub 2005 Nov 28. Plant Cell. 2006. PMID: 16314447 Free PMC article. - The Fungal Protein Mes1 Is Required for Morphogenesis and Virulence in the Dimorphic Phytopathogen Ustilago maydis.
Cánovas D. Cánovas D. J Fungi (Basel). 2022 Jul 22;8(8):759. doi: 10.3390/jof8080759. J Fungi (Basel). 2022. PMID: 35893127 Free PMC article. - The functions of myosin II and myosin V homologs in tip growth and septation in Aspergillus nidulans.
Taheri-Talesh N, Xiong Y, Oakley BR. Taheri-Talesh N, et al. PLoS One. 2012;7(2):e31218. doi: 10.1371/journal.pone.0031218. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359575 Free PMC article.
References
- Ayscough, K.R., and Drubin, D.G. (1998). A role for the yeast actin cytoskeleton in pheromone receptor clustering and signalling. Curr. Biol. 8, 927–930. - PubMed
- Banuett, F. (1995). Genetics of Ustilago maydis, a fungal pathogen that induces tumors in maize. Annu. Rev. Genet. 29, 179–208. - PubMed
- Banuett, F., and Herskowitz, I. (1996). Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development 122, 2965–2976. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous