Antithrombotic activity of TNF-alpha - PubMed (original) (raw)
Antithrombotic activity of TNF-alpha
Beatrice Cambien et al. J Clin Invest. 2003 Nov.
Abstract
Basic and clinical observations suggest that thrombosis and inflammation are closely related. Here we addressed the role played by TNF-alpha in thrombus formation and growth in an in vivo mouse model. Using intravital microscopy, we show that systemic administration of TNF-alpha at doses found in sepsis transiently inhibits thrombus formation and delays arterial occlusion upon vascular injury. These results were reflected in a prolonged bleeding time. Platelets isolated from the TNF-alpha-treated mice showed a marked decrease in fibrinogen binding and P-selectin expression as well as reduced platelet aggregation in response to various agonists. In contrast, in vitro treatment of platelets with TNF-alpha did not affect their function. TNF receptor 1- and 2-deficient mice exhibited normal thrombogenesis in the presence of TNF-alpha. Additionally, the inhibitory effect of TNF-alpha was lost either after treatment with NG-monomethyl-l-arginine, an inhibitor of NO production, or in mice deficient for iNOS. These results indicate that under inflammatory conditions, when leukocytes need free passage to transmigrate into tissues, TNF-alpha decreases platelet activation and inhibits thrombi formation. This effect is not exerted directly on platelets but mediated through the rapid generation of NO in the vessel wall.
Figures
Figure 1
Effect of TNF-α on thrombus formation in vivo. Thrombus formation in response to vascular injury was visualized in arterioles of control (upper) or TNF-α–treated mice (lower). The different lengths of time after ferric chloride application are indicated. No significant difference in initial platelet adhesion to the injured vessel was observed between the two groups (3 minutes). The appearance of thrombi was delayed by TNF-α infusion (8 minutes), however. In addition, thrombi in treated mice were unstable and often did not grow to occlusive size, leaving, after 14 minutes, a still patent vessel, whereas the control arterioles occluded. n = 12–15 mice.
Figure 2
Quantitative analysis of arterial thrombogenesis in WT, TNF-R1/2–/–,
L
-NMMA–treated, and iNOS–/– mice. Two minutes after the injury, the number of platelet-vessel wall interactions in each group of mice was comparable between the TNF-α–treated and untreated animals (a–d). In contrast to the antithrombotic effect measured in WT mice (a), TNF-α did not significantly affect thrombus formation and vessel occlusion in _TNF-R1/2_–/– (b),
L
–NMMA–treated (c), and iNOS_–/– mice (d). Compared with WT mice, iNOS_–/– mice exhibited a significant increase in platelet deposition (P < 0.02) and a slightly shorter time in thrombus formation (P < 0.05) and in vessel occlusion (P < 0.03). n = 7–10 mice per group. *P < 0.05; **P < 0.005 versus untreated WT.
Figure 3
Tail bleeding time and plasma clotting time in TNF-α–treated mice. Mice 12–16 weeks old were infused with PBS or TNF-α. (a) The bleeding time was measured after amputation of a 3-mm portion of the tail. The mice injected with TNF-α (filled circles) exhibited a fourfold prolonged bleeding time compared with control mice (open circles) (P < 0.003). (b) Plasma clotting time was measured in an aggregometer after adding CaCl2 solution. The clotting time was significantly shorter in mice treated with 1 ng/ml of TNF-α (black bar) than in control mice (white bar). P < 0.001, n = 8 mice per group.
Figure 4
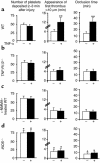
Effect of in vivo and in vitro treatments with TNF-α on platelet function. PBS (white bars) or TNF-α (black bars) were administered for 30 minutes either to whole blood samples (a, c, and e) or by intravenous injection into mice (b, d, and f). PRP was prepared, and platelets were tested for aggregation in response to 2 μM ADP (e and f). To study platelet activation by flow cytometry, platelets were washed, activated for 5 minutes with thrombin (0.1 U/ml) or CRP (2 μg/ml), and incubated for 10 minutes at 37°C with FITC-conjugated Ab’s against human fibrinogen (a and b) or a FITC-conjugated mAb against P-selectin (c and d). (g and h) Shown are representative results of TNF-α systemic administration on fibrinogen binding and platelet aggregation. n = 6 mice per group. *P < 0.05; **P < 0.005. MFI, mean fluorescence intensity.
Similar articles
- Stimulation of platelet nitric oxide production by nebivolol prevents thrombosis.
Momi S, Caracchini R, Falcinelli E, Evangelista S, Gresele P. Momi S, et al. Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):820-9. doi: 10.1161/ATVBAHA.114.303290. Epub 2014 Feb 20. Arterioscler Thromb Vasc Biol. 2014. PMID: 24558107 - Antithrombotic properties of water-soluble carbon monoxide-releasing molecules.
Kramkowski K, Leszczynska A, Mogielnicki A, Chlopicki S, Fedorowicz A, Grochal E, Mann B, Brzoska T, Urano T, Motterlini R, Buczko W. Kramkowski K, et al. Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2149-57. doi: 10.1161/ATVBAHA.112.253989. Epub 2012 Jul 5. Arterioscler Thromb Vasc Biol. 2012. PMID: 22772756 - The many antithrombotic actions of nitric oxide.
Tziros C, Freedman JE. Tziros C, et al. Curr Drug Targets. 2006 Oct;7(10):1243-51. doi: 10.2174/138945006778559111. Curr Drug Targets. 2006. PMID: 17073585 Review. - Role of Endothelial Cells in Acute and Chronic Thrombosis.
Bochenek ML, Schäfer K. Bochenek ML, et al. Hamostaseologie. 2019 Jun;39(2):128-139. doi: 10.1055/s-0038-1675614. Epub 2019 Jan 8. Hamostaseologie. 2019. PMID: 30620989 Review.
Cited by
- Autoantibodies and thrombophilia in RA: TNFalpha and TNFalpha blockers.
Ferraccioli GF, Gremese E. Ferraccioli GF, et al. Ann Rheum Dis. 2004 Jun;63(6):613-5. doi: 10.1136/ard.2003.015586. Epub 2004 Mar 17. Ann Rheum Dis. 2004. PMID: 15028556 Free PMC article. No abstract available. - Prothrombotic effects of tumor necrosis factor alpha in vivo are amplified by the absence of TNF-alpha receptor subtype 1 and require TNF-alpha receptor subtype 2.
Pircher J, Merkle M, Wörnle M, Ribeiro A, Czermak T, Stampnik Y, Mannell H, Niemeyer M, Vielhauer V, Krötz F. Pircher J, et al. Arthritis Res Ther. 2012 Oct 18;14(5):R225. doi: 10.1186/ar4064. Arthritis Res Ther. 2012. PMID: 23079185 Free PMC article. - Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets.
Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K, Shin Y, Hirashima M, Ozaki Y. Suzuki-Inoue K, et al. J Biol Chem. 2010 Aug 6;285(32):24494-507. doi: 10.1074/jbc.M110.130575. Epub 2010 Jun 4. J Biol Chem. 2010. PMID: 20525685 Free PMC article. - Signaling-mediated functional activation of inducible nitric-oxide synthase and its role in stimulating platelet activation.
Marjanovic JA, Stojanovic A, Brovkovych VM, Skidgel RA, Du X. Marjanovic JA, et al. J Biol Chem. 2008 Oct 24;283(43):28827-34. doi: 10.1074/jbc.M801646200. Epub 2008 Aug 27. J Biol Chem. 2008. PMID: 18753139 Free PMC article. - Cardiac failure in C5-deficient A/J mice after Candida albicans infection.
Mullick A, Leon Z, Min-Oo G, Berghout J, Lo R, Daniels E, Gros P. Mullick A, et al. Infect Immun. 2006 Aug;74(8):4439-51. doi: 10.1128/IAI.00159-06. Infect Immun. 2006. PMID: 16861630 Free PMC article.
References
- Vallet B, Wiel E. Endothelial cell dysfunction and coagulation. Crit. Care Med. 2001;29(Suppl.):S36–S41. - PubMed
- Ruberg FL, Leopold JA, Loscalzo J. Atherothrombosis: plaque instability and thrombogenesis. Prog. Cardiovasc. Dis. 2002;44:381–394. - PubMed
- Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002;3:27–34. - PubMed
- Korte W. Veno-occlusive disease of the liver after bone marrow transplantation: is hypercoagulability really part of the problem? Blood Coagul. Fibrinolysis. 1997;8:367–381. - PubMed
- Esmon CT. Possible involvement of cytokines in diffuse intravascular coagulation and thrombosis. Baillieres Best Pract. Res. Clin. Haematol. 1999;12:343–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37-HL-41002/HL/NHLBI NIH HHS/United States
- R01-HL-53756/HL/NHLBI NIH HHS/United States
- R37 HL041002/HL/NHLBI NIH HHS/United States
- R01 HL053756/HL/NHLBI NIH HHS/United States
- P01 HL056949/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases