A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting - PubMed (original) (raw)
Comparative Study
. 2003 Dec;73(6):1293-301.
doi: 10.1086/380418. Epub 2003 Nov 18.
Affiliations
- PMID: 14628289
- PMCID: PMC1180395
- DOI: 10.1086/380418
Comparative Study
A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting
Dominik Müller et al. Am J Hum Genet. 2003 Dec.
Abstract
Mutations in the gene coding for the renal tight junction protein claudin 16 cause familial hypomagnesemia with hypercalciuria and nephrocalcinosis, an autosomal recessive disorder of renal Ca(2+) and Mg(2+) handling that progressively leads to chronic renal failure, with nephrolithiasis having been reported in heterozygous carriers. Screening a cohort of 11 families with idiopathic hypercalciuria identified a novel homozygous mutation in the claudin 16 gene in two families. In contrast to classical symptoms of familial hypomagnesemia with hypercalciuria and nephrocalcinosis, the patients displayed serious but self-limiting childhood hypercalciuria with preserved glomerular filtration rate. The mutation results in inactivation of a PDZ-domain binding motif, thereby disabling the association of the tight junction scaffolding protein ZO-1 with claudin 16. In contrast to wild-type claudin 16, the mutant no longer localizes to tight junctions in kidney epithelial cells but instead accumulates in lysosomes. Thus, mutations at different intragenic sites in the claudin 16 gene may lead to particular clinical phenotypes with a distinct prognosis. Mutations in claudin 16 that affect interaction with ZO-1 lead to lysosomal mistargeting, providing-for the first time, to our knowledge-insight into the molecular mechanism of a disease-associated mutation in the claudin 16 gene.
Figures
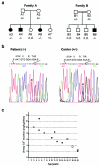
Figure 1
Clinical and molecular data regarding patients from two families with infantile familial HC with NC. a, Pedigrees of patient families. Blackened symbols indicate affected individuals; unblackened symbols indicate nonaffected individuals; squares indicate males; circles indicate females. Family B is consanguineous (see text for details). b, Sequencing data for the region in the CLDN16 gene carrying the T233R mutation. Homozygous (−/−) amino acid exchange (T→R) was detected in affected patients A3, A4, A6, and B6; heterozygous (+/−) mutations were detected in the nonaffected members A1, A2, A7, B1, and B2. Individuals B3, B4, and B5 were not available for analysis (“n.d.”). Sequences in the chromatogram read from 3′ to 5′, and the mutation is indicated with an asterisk (*). The coding and corresponding amino acid sequences are provided above the chromatogram. c, Urinary Ca2+ excretion decreases to levels within the normal range when patients reach adolescence. Ca2+ excretion was measure in affected members of families A and B during follow-up.
Figure 2
Predicted topology of CLDN16 and location of the different mutations reported. Shown is the amino acid sequence from the second translation-initiation site, which is used in vivo (Ohba et al. ; Weber et al. 2001_a,_ 2001_b_). Mutated amino acids thus far reported in the literature that are associated with classical FHHNC are shown (Simon et al. ; Weber et al. ; Weber et al. 2001_b_) (yellow), with the G128A mutation characterized in this study highlighted (blue). The C-terminal PDZ binding motif (red) and the novel T233R mutation encountered in the patients of families A and B (blue) are also shown. Tyrosine residues in the C-terminal cytosolic domain of CLDN16 that may be part of endocytosis and/or lysosomal sorting signals are indicated (gray).
Figure 3
Binding of wild-type and mutant CLDN16 to ZO-1. a, Wild-type CLDN16, but not the T233R mutant, binds to the ZO-1 but not the ZO-2 and ZO-3 PDZ domains. In vitro translated and radioactively labeled ZO-1, ZO-2, or ZO-3 PDZ domains were incubated with peptides corresponding to the C-terminus of wild-type or T233R mutant CLDN16 coupled to beads. Protein bound to the beads (“Pull-Down”) was analyzed by SDS-PAGE and autoradiography, and an aliquot (5%) of the in vitro translated material was directly analyzed to confirm that similar amounts of the in vitro translated proteins were added to the binding reaction (“Input”). b, Preincubation with wild-type, but not T233R mutant, peptides competes with ZO-1 PDZ binding to beads. In vitro translated and radioactively labeled ZO-1 PDZ domains were pre-incubated with soluble wild-type or mutant CLDN16 peptides prior to the incubation with the peptide-coupled beads. c, Wild-type CLDN16, but not the T233R mutant, coprecipitates with ZO-1. Control cells (MDCK) or cells stably expressing wild-type (CLDN16) or mutant (T233R) CLDN16 were lysed, and equal amounts of total protein were used to immunoprecipitate CLDN16. Precipitates were blotted with anti–ZO-1 antibody to detect ZO-1 bound to CLDN16. In the reverse experiment, wild-type, but not mutant CLDN16, which is also associated with anti–ZO-1, precipitates (data not shown). Aliquots of the cell lysate were blotted directly to confirm that the cells expressed similar amounts of ZO-1, as well as wild-type and mutant CLDN16 (“Input”). WT = wild type.
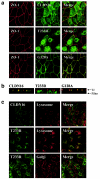
Figure 4
Subcellular localization of wild-type and mutant CLDN16. a, Colocalization of CLDN16 and ZO-1 is abolished by the T233R mutation. MDCK cells stably expressing wild-type (CLDN16) or mutant (T233R or G128A) CLDN16 grown as polarized cell monolayers on polycarbonate filters were stained for ZO-1 (red) and CLDN16 (green), and confocal images corresponding to horizontal sections at the height of TJ were acquired. The merged image shows regions where CLDN16 and ZO-1 colocalize (yellow). b, TJ localization of CLDN16 is abolished by the T233R mutation. Vertical confocal sections along the apico-basal axis of the cell monolayers expressing wild-type (CLDN16) or mutant (T233R or G128A) CLDN16 labeled for CLDN16 (green) or ZO-1 (red). The positions of the filter supporting the cells and the TJ are indicated. c, T233R localized to lysosomes. MDCK cells transfected with wild-type (CLDN16) or mutant (T233R) CLDN16 were stained to visualize lysosomes (anti–lamp-2) or the Golgi complex (anti-GM130) (red) and CLDN16 (green), and confocal images were acquired. The T223R mutant shows extensive localization to lyososmes (merged image; yellow), but not the Golgi complex.
Similar articles
- Characterization of two novel mutations in the claudin-16 and claudin-19 genes that cause familial hypomagnesemia with hypercalciuria and nephrocalcinosis.
Perdomo-Ramirez A, Aguirre M, Davitaia T, Ariceta G, Ramos-Trujillo E; RenalTube Group; Claverie-Martin F. Perdomo-Ramirez A, et al. Gene. 2019 Mar 20;689:227-234. doi: 10.1016/j.gene.2018.12.024. Epub 2018 Dec 18. Gene. 2019. PMID: 30576809 - Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins.
Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Itoh M, et al. J Cell Biol. 1999 Dec 13;147(6):1351-63. doi: 10.1083/jcb.147.6.1351. J Cell Biol. 1999. PMID: 10601346 Free PMC article. - Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis.
Weber S, Schneider L, Peters M, Misselwitz J, Rönnefarth G, Böswald M, Bonzel KE, Seeman T, Suláková T, Kuwertz-Bröking E, Gregoric A, Palcoux JB, Tasic V, Manz F, Schärer K, Seyberth HW, Konrad M. Weber S, et al. J Am Soc Nephrol. 2001 Sep;12(9):1872-1881. doi: 10.1681/ASN.V1291872. J Am Soc Nephrol. 2001. PMID: 11518780 - An unusual patient with hypercalciuria, recurrent nephrolithiasis, hypomagnesemia and G227R mutation of Paracellin-1. An unusual patient with hypercalciuria and hypomagnesemia unresponsive to thiazide diuretics.
Kutluturk F, Temel B, Uslu B, Aral F, Azezli A, Orhan Y, Konrad M, Ozbey N. Kutluturk F, et al. Horm Res. 2006;66(4):175-81. doi: 10.1159/000094253. Epub 2006 Jun 27. Horm Res. 2006. PMID: 16804318 Review. - Claudins and mineral metabolism.
Hou J. Hou J. Curr Opin Nephrol Hypertens. 2016 Jul;25(4):308-13. doi: 10.1097/MNH.0000000000000239. Curr Opin Nephrol Hypertens. 2016. PMID: 27191348 Free PMC article. Review.
Cited by
- tRNA N6-adenosine threonylcarbamoyltransferase defect due to KAE1/TCS3 (OSGEP) mutation manifest by neurodegeneration and renal tubulopathy.
Edvardson S, Prunetti L, Arraf A, Haas D, Bacusmo JM, Hu JF, Ta-Shma A, Dedon PC, de Crécy-Lagard V, Elpeleg O. Edvardson S, et al. Eur J Hum Genet. 2017 May;25(5):545-551. doi: 10.1038/ejhg.2017.30. Epub 2017 Mar 8. Eur J Hum Genet. 2017. PMID: 28272532 Free PMC article. - Common variants in CLDN14 are associated with differential excretion of magnesium over calcium in urine.
Corre T, Olinger E, Harris SE, Traglia M, Ulivi S, Lenarduzzi S, Belge H, Youhanna S, Tokonami N, Bonny O, Houillier P, Polasek O, Deary IJ, Starr JM, Toniolo D, Gasparini P, Vollenweider P, Hayward C, Bochud M, Devuyst O. Corre T, et al. Pflugers Arch. 2017 Jan;469(1):91-103. doi: 10.1007/s00424-016-1913-7. Epub 2016 Dec 3. Pflugers Arch. 2017. PMID: 27915449 Review. - Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement.
Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Konrad M, et al. Am J Hum Genet. 2006 Nov;79(5):949-57. doi: 10.1086/508617. Epub 2006 Sep 19. Am J Hum Genet. 2006. PMID: 17033971 Free PMC article. - The carboxy-terminus, a key regulator of protein function.
Sharma S, Schiller MR. Sharma S, et al. Crit Rev Biochem Mol Biol. 2019 Apr;54(2):85-102. doi: 10.1080/10409238.2019.1586828. Epub 2019 May 20. Crit Rev Biochem Mol Biol. 2019. PMID: 31106589 Free PMC article. Review. - Do cell junction protein mutations cause an airway phenotype in mice or humans?
Chang EH, Pezzulo AA, Zabner J. Chang EH, et al. Am J Respir Cell Mol Biol. 2011 Aug;45(2):202-20. doi: 10.1165/rcmb.2010-0498TR. Epub 2011 Feb 4. Am J Respir Cell Mol Biol. 2011. PMID: 21297078 Free PMC article. Review.
References
Electronic-Database Information
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for CLDN16 sequence [accession number AF152101] and oligonucleotides [accession number NT005883])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for FHHNC) - PubMed
References
- Alon U, Warady BA, Hellerstein S (1990) Hypercalciuria in the frequency-dysuria syndrome of childhood. J Pediatr 116:103–105 - PubMed
- Asplin JR, Favus MJ, Coe FL (1996) In: Brenner BM (ed). The kidney, ed. 5. Philadelphia, W. B. Saunders, pp 1893–1935
- Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P (2001) Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int 59:2206–221510.1046/j.1523-1755.2001.0590062206.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous