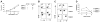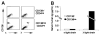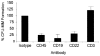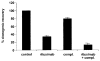Characterization of clonogenic multiple myeloma cells - PubMed (original) (raw)
Characterization of clonogenic multiple myeloma cells
William Matsui et al. Blood. 2004.
Abstract
The identity of the cells responsible for the initiation and maintenance of multiple myeloma (MM) remains unclear largely because of the difficulty growing MM cells in vitro and in vivo. MM cell lines and clinical specimens are characterized by malignant plasma cells that express the cell surface antigen syndecan-1 (CD138); however, CD138 expression is limited to terminally differentiated plasma cells during B-cell development. Moreover, circulating B cells that are clonally related to MM plasma cells have been reported in some patients with MM. We found that human MM cell lines contained small (< 5%) subpopulations that lacked CD138 expression and had greater clonogenic potential in vitro than corresponding CD138+ plasma cells. CD138- cells from clinical MM samples were similarly clonogenic both in vitro and in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice, whereas CD138+ cells were not. Furthermore, CD138- cells from both cell lines and clinical samples phenotypically resembled postgerminal center B cells, and their clonogenic growth was inhibited by the anti-CD20 monoclonal antibody rituximab. These data suggest that MM "stem cells" are CD138- B cells with the ability to replicate and subsequently differentiate into malignant CD138+ plasma cells.
Figures
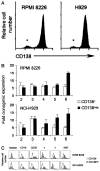
Figure 1. Clonogenicity of MM cell lines by CD138 expression
(A) Flow cytometric evaluation of CD138 expression by RPMI 8226 (λ light chain restricted) and NCI-H929 cells (κ light chain restricted). * denotes CD138− population. (B) Clonogenic expansion of CD138+ and CD138− RPMI 8226 and NCI-H929 cells during serial replating. Results are the mean ± SEM of 3 separate experiments for each cell line and represent the fold clonogenic expansion expressed as a ratio of number of colonies scored compared to the previous plating. P < .02 following the fifth and sixth transfer of both cell lines by Student t test. X-axis represents round of serial replating. (C) Flow cytometric evaluation comparing additional antigens expressed by CD138+ and CD138− RPMI 8226 and NCI-H929 cells.
Figure 2. Clonogenic growth of MM cells from clinical specimens
(A) MM colony formation of CD138+ and CD138− cells from 3 clinical samples plated at various densities. (B) Flow cytometric evaluation of colonies derived from CD138−/CD34− cells. The top 2 panels show the initial clinical marrow specimen; the middle panels, the CD138-depleted cells prior to plating; and the bottom panels, the pooled colonies after 21 days from a representative MM patient. (C) Flow cytometric evaluation of pooled colonies from 3 additional patients. (D) Colony formation of CD138− clinical MM samples during serial replating.
Figure 3. Engraftment of MM CD138− cells in NOD/SCID mice
(A) Flow cytometric evaluation of murine bone marrow from animals injected with CD138−/CD34− or CD138+/CD34− cells from a single patient. (B) Serum human immunoglobulin levels measured by ELISA in mice injected with CD138−/CD34− or CD138+/CD34− cells from a single patient.
Figure 4. MM colony formation of CD138−/CD34− cells following antibody-mediated cell depletion
Results are presented as a percentage of the untreated control group that was depleted of CD34+ and CD138+ cells. P < .01 for CD45+, CD19+, and CD22+ cell depletions calculated by Student t test comparing each group with the untreated control (n = 12). CFU indicates colony-forming units.
Figure 5. Effect of rituximab on clonogenic MM progenitors in vitro
MM colony formation following treatment of bone marrow mononuclear cells depleted of CD34+ and CD138+ cells with rituximab or 10% human serum (compl.). Results are presented as a percentage of the untreated control group. P < .01 for the rituximab and rituximab plus complement groups compared to the untreated (control) group by Student t test (n = 3).
Similar articles
- Leukemic B cells clonally identical to myeloma plasma cells are myelomagenic in NOD/SCID mice.
Pilarski LM, Seeberger K, Coupland RW, Eshpeter A, Keats JJ, Taylor BJ, Belch AR. Pilarski LM, et al. Exp Hematol. 2002 Mar;30(3):221-8. doi: 10.1016/s0301-472x(01)00788-3. Exp Hematol. 2002. PMID: 11882359 - Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance.
Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, McNiece I, Lin L, Ambinder RF, Peacock C, Watkins DN, Huff CA, Jones RJ. Matsui W, et al. Cancer Res. 2008 Jan 1;68(1):190-7. doi: 10.1158/0008-5472.CAN-07-3096. Cancer Res. 2008. PMID: 18172311 Free PMC article. - Cytotoxic activity of the maytansinoid immunoconjugate B-B4-DM1 against CD138+ multiple myeloma cells.
Tassone P, Goldmacher VS, Neri P, Gozzini A, Shammas MA, Whiteman KR, Hylander-Gans LL, Carrasco DR, Hideshima T, Shringarpure R, Shi J, Allam CK, Wijdenes J, Venuta S, Munshi NC, Anderson KC. Tassone P, et al. Blood. 2004 Dec 1;104(12):3688-96. doi: 10.1182/blood-2004-03-0963. Epub 2004 Aug 3. Blood. 2004. PMID: 15292058 - Syndecan-1 in B lymphoid malignancies.
Sanderson RD, Børset M. Sanderson RD, et al. Ann Hematol. 2002 Mar;81(3):125-35. doi: 10.1007/s00277-002-0437-8. Epub 2002 Mar 1. Ann Hematol. 2002. PMID: 11904737 Review. - Multiple myeloma-initiating cells.
Hosen N. Hosen N. Int J Hematol. 2013 Mar;97(3):306-12. doi: 10.1007/s12185-013-1293-0. Epub 2013 Feb 19. Int J Hematol. 2013. PMID: 23420183 Review.
Cited by
- Can we change the disease biology of multiple myeloma?
Borrello I. Borrello I. Leuk Res. 2012 Nov;36 Suppl 1(0 1):S3-12. doi: 10.1016/S0145-2126(12)70003-6. Leuk Res. 2012. PMID: 23176722 Free PMC article. Review. - CAR T-Cells in Multiple Myeloma: State of the Art and Future Directions.
Rodríguez-Lobato LG, Ganzetti M, Fernández de Larrea C, Hudecek M, Einsele H, Danhof S. Rodríguez-Lobato LG, et al. Front Oncol. 2020 Jul 28;10:1243. doi: 10.3389/fonc.2020.01243. eCollection 2020. Front Oncol. 2020. PMID: 32850376 Free PMC article. Review. - Small molecule antibody targeting HLA class I inhibits myeloma cancer stem cells by repressing pluripotency-associated transcription factors.
Ikegame A, Ozaki S, Tsuji D, Harada T, Fujii S, Nakamura S, Miki H, Nakano A, Kagawa K, Takeuchi K, Abe M, Watanabe K, Hiasa M, Kimura N, Kikuchi Y, Sakamoto A, Habu K, Endo M, Itoh K, Yamada-Okabe H, Matsumoto T. Ikegame A, et al. Leukemia. 2012 Sep;26(9):2124-34. doi: 10.1038/leu.2012.78. Epub 2012 Mar 20. Leukemia. 2012. PMID: 22430632 - Bruton tyrosine kinase inhibition is a novel therapeutic strategy targeting tumor in the bone marrow microenvironment in multiple myeloma.
Tai YT, Chang BY, Kong SY, Fulciniti M, Yang G, Calle Y, Hu Y, Lin J, Zhao JJ, Cagnetta A, Cea M, Sellitto MA, Zhong MY, Wang Q, Acharya C, Carrasco DR, Buggy JJ, Elias L, Treon SP, Matsui W, Richardson P, Munshi NC, Anderson KC. Tai YT, et al. Blood. 2012 Aug 30;120(9):1877-87. doi: 10.1182/blood-2011-12-396853. Epub 2012 Jun 11. Blood. 2012. PMID: 22689860 Free PMC article. - Stem cell niche dynamics: from homeostasis to carcinogenesis.
Tieu KS, Tieu RS, Martinez-Agosto JA, Sehl ME. Tieu KS, et al. Stem Cells Int. 2012;2012:367567. doi: 10.1155/2012/367567. Epub 2012 Feb 9. Stem Cells Int. 2012. PMID: 22448171 Free PMC article.
References
- Drewinko B, Alexanian R, Boyer H, Barlogie B, Rubinow SI. The growth fraction of human myeloma cells. Blood. 1981;57:333–338. - PubMed
- Pilarski LM, Jensen GS. Monoclonal circulating B cells in multiple myeloma: a continuously differentiating, possibly invasive, population as defined by expression of CD45 isoforms and adhesion molecules. Hematol Oncol Clin North Am. 1992;6:297–322. - PubMed
- Bakkus MH, Van RI, Van Camp B, Thielemans K. Evidence that the clonogenic cell in multiple myeloma originates from a pre-switched but somatically mutated B cell. Br J Haematol. 1994;87:68–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01CA70970/CA/NCI NIH HHS/United States
- P01 CA015396/CA/NCI NIH HHS/United States
- P01CA15396/CA/NCI NIH HHS/United States
- P01 CA070970/CA/NCI NIH HHS/United States
- R01 CA127574/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous