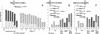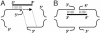Chromosomal site-specific double-strand breaks are efficiently targeted for repair by oligonucleotides in yeast - PubMed (original) (raw)
Chromosomal site-specific double-strand breaks are efficiently targeted for repair by oligonucleotides in yeast
Francesca Storici et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The repair of chromosomal double-strand breaks (DSBs) can be accomplished through homologous recombination in most organisms. We report here that exogenous oligonucleotides can efficiently target for repair a single DSB induced in a chromosome of yeast. The efficiency of recombinational targeting leading to a desired DNA change can be as high as 20% of cells. The DSB was generated either by a regulatable I-SceI endonuclease just before transformation or appeared spontaneously at the site of a long inverted repeat composed of human Alu sequences. The approach used features of our previously described delitto perfetto system for selecting transformants with integrative recombinant oligonucleotides. The DSB repair mediated by pairs of complementary integrative recombinant oligonucleotides was efficient for targeting to homologous sequences that were close to or distant from the DSB and in the presence of a competing homologous chromosome in diploid cells. We also demonstrate that a DSB can strongly stimulate recombination with single-stranded DNA, without strand bias. These findings expand current models of DSB repair. In addition, we establish a high-throughput system for rapid genome-wide modification with oligonucleotides.
Figures
Fig. 1.
A self-contained inducible DSB system for the targeted integration of oligonucleotides into the yeast genome. Presented is a scheme for the PCR amplification and genomic integration of the CORE-I-_Sce_I cassette. A segment of chromosome VII is shown with the CORE-I-_Sce_I and a DSB induced at the I-_Sce_I site within the TRP5 locus. Categories of IROs are defined by the position of the homologous tails relative to the position of the DSB. IROs that target to both sides of the break provide an opportunity for repair (R). They are classified as repair-adjacent distant (R-AD), repair-distant adjacent (R-DA), repair-distant distant (R-DD), or repair-adjacent adjacent (R-AA) (see Figs. 2, 3, and 5), according to whether the homology region is adjacent (A) (<15 bp from the I-_Sce_I site) or distant (D) (up to 4.7 kb from one side and 11.9 kb from the other) to the DSB. Recombination with IROs during transformation leads to excision of the CORE-I-_Sce_I cassette and the appearance of colonies with the desired mutation (asterisks) or deletion.
Fig. 2.
Factors affecting IRO targeting efficiency to a DSB (structure and homology of IROs relative to the position of the DSB). Presented are numbers of Trp+ transformants per 107 viable cells resulting from targeting by the various IROs to an I-_Sce_I DSB induced in the TRP5 gene. The vertical bars correspond to the average values from three to six determinations; the range identifies the standard deviations. Dark gray bars represent values from pairs of fully complementary IROs, medium gray bars represent values from pairs of overlapping IROs, and light gray bars represent values for ss IROs. When no bars are present, values were ≤1 transformant/107 viable cells. “No-DSB” control values (corresponding to cells incubated in glucose media only) are indicated by stippled bars. The types of IROs analyzed are indicated below the bars. The amount of total IRO DNA used in each transformation is 1 nmol (≈10-30 μg) unless otherwise indicated. The IROs examined in A are referred to as e and f: fully complementary with equal lengths of homology to both sides of the DSB (i.e., symmetrical for homology) and from 31 to 95 nucleotides in length. The oligonucleotides examined in B and C are various combinations of 80-mers referred to as a, b, c, and d, where the homology to each side of the DSB is asymmetric (i.e., 69 nucleotides to one side and 10 nucleotides to the other). The position of the CORE-I-_Sce_I cassette and the DSB in TRP5, together with the kinds of IROs used, are shown in the small diagrams. In A and B, the DSB is between the 5′ region of TRP5 and the CORE-I-_Sce_I cassette; in C, the DSB is between the CORE-I-_Sce_I cassette and the 3′ region of TRP5. (A) Effect of size of fully complementary and ss e and f IROs on transformation efficiency in the R-AD conformation. (B and C) Effect of positions of homology relative to the DSB, using the asymmetric a, b, c, and d IROs in the R-AD and R-DA conformation, respectively.
Fig. 3.
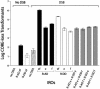
Effect on IRO targeting of position of homology relative to a DSB. Presented are numbers of 5-FOAR/HygroS transformants per 107 viable cells resulting from targeting to a DSB induced in the TRP5 gene by oligonucleotides that are fully complementary and symmetrical for homology (equal size of target sequences; see Fig. 1 and Table 2). Shown are the e and f R-AD oligonucleotides (81-mers that restore TRP5) and the R-DD oligonucleotides (83-mers that delete the CORE-I-_Sce_I cassette plus 11.9 kb around the TRP5 locus). Vertical bars along with the standard deviations correspond to the average values from three to six determinations. Black, gray, and white bars are values for R-AD, R-AD + R-DD, and R-DD IROs, respectively. The striped bar represents the no-DNA control in galactose. The no-DNA control value (for conditions of no-DSB induction) is ≤1 transformant/107 viable cells. The types of IROs analyzed are indicated below the bars, and the amount of total IRO DNA used in each transformation is 1 nmol.
Fig. 4.
Models of IRO targeted interactions and repair of a DSB. Homologous pairing interactions between the ss oligonucleotide (thick line) and the DSB ends are presented as short thin parallel vertical lines. (A) Template intermediate model. The IRO pairs with the homologous region on the 3′ strand after resection of the 5′ strand. The small arrow identifies the position at which nonhomologous sequence is cut. DNA synthesis (dotted line) can occur to copy the rest of the oligonucleotide. Homologous sequences between the 3′ ends of the DSB appear between the thin dotted lines. (B) Bridge intermediate model. As a result of unwinding of the DSB ends, annealing of the oligonucleotide can occur with the homologous regions on either side of the DSB creating a “bridge” between the two ends.
Similar articles
- A Rad51-independent pathway promotes single-strand template repair in gene editing.
Gallagher DN, Pham N, Tsai AM, Janto NV, Choi J, Ira G, Haber JE. Gallagher DN, et al. PLoS Genet. 2020 Oct 15;16(10):e1008689. doi: 10.1371/journal.pgen.1008689. eCollection 2020 Oct. PLoS Genet. 2020. PMID: 33057349 Free PMC article. - Conservative repair of a chromosomal double-strand break by single-strand DNA through two steps of annealing.
Storici F, Snipe JR, Chan GK, Gordenin DA, Resnick MA. Storici F, et al. Mol Cell Biol. 2006 Oct;26(20):7645-57. doi: 10.1128/MCB.00672-06. Epub 2006 Aug 14. Mol Cell Biol. 2006. PMID: 16908537 Free PMC article. - Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae.
Pâques F, Haber JE. Pâques F, et al. Microbiol Mol Biol Rev. 1999 Jun;63(2):349-404. doi: 10.1128/MMBR.63.2.349-404.1999. Microbiol Mol Biol Rev. 1999. PMID: 10357855 Free PMC article. Review. - Using nucleases to stimulate homologous recombination.
Carroll D. Carroll D. Methods Mol Biol. 2004;262:195-207. doi: 10.1385/1-59259-761-0:195. Methods Mol Biol. 2004. PMID: 14769963 Review.
Cited by
- Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells.
Hoban MD, Cost GJ, Mendel MC, Romero Z, Kaufman ML, Joglekar AV, Ho M, Lumaquin D, Gray D, Lill GR, Cooper AR, Urbinati F, Senadheera S, Zhu A, Liu PQ, Paschon DE, Zhang L, Rebar EJ, Wilber A, Wang X, Gregory PD, Holmes MC, Reik A, Hollis RP, Kohn DB. Hoban MD, et al. Blood. 2015 Apr 23;125(17):2597-604. doi: 10.1182/blood-2014-12-615948. Epub 2015 Mar 2. Blood. 2015. PMID: 25733580 Free PMC article. - A mechanism of gene amplification driven by small DNA fragments.
Mukherjee K, Storici F. Mukherjee K, et al. PLoS Genet. 2012;8(12):e1003119. doi: 10.1371/journal.pgen.1003119. Epub 2012 Dec 13. PLoS Genet. 2012. PMID: 23271978 Free PMC article. - Mating-type genes and MAT switching in Saccharomyces cerevisiae.
Haber JE. Haber JE. Genetics. 2012 May;191(1):33-64. doi: 10.1534/genetics.111.134577. Genetics. 2012. PMID: 22555442 Free PMC article. Review. - Genes with a Combination of Over-Dominant and Epistatic Effects Underlie Heterosis in Growth of Saccharomyces cerevisiae at High Temperature.
Shapira R, David L. Shapira R, et al. Front Genet. 2016 May 4;7:72. doi: 10.3389/fgene.2016.00072. eCollection 2016. Front Genet. 2016. PMID: 27200081 Free PMC article. - CRISPR system in the yeast Saccharomyces cerevisiae and its application in the bioproduction of useful chemicals.
Mitsui R, Yamada R, Ogino H. Mitsui R, et al. World J Microbiol Biotechnol. 2019 Jul 6;35(7):111. doi: 10.1007/s11274-019-2688-8. World J Microbiol Biotechnol. 2019. PMID: 31280424 Review.
References
- Resnick, M. A. (1978) J. Theor. Biol. 71, 339-346. - PubMed
- Pfeiffer, P., Goedecke, W. & Obe, G. (2000) Mutagenesis 15, 289-302. - PubMed
- Jackson, S. P. (2002) Carcinogenesis 23, 687-696. - PubMed
- Pastink, A., Eeken, J. C. & Lohman, P. H. (2001) Mutat. Res. 480-481, 37-50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials