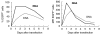Cancer immunotherapy using RNA-loaded dendritic cells - PubMed (original) (raw)
Review
Cancer immunotherapy using RNA-loaded dendritic cells
P Ponsaerts et al. Clin Exp Immunol. 2003 Dec.
Abstract
Dendritic cells (DC) are the most professional antigen-presenting cells of the immune system and are capable of initiating immune responses in vitro and in vivo. One of the great challenges in immunotherapy protocols is to introduce relevant antigens into DC for stimulation of major histocompatibility complex (MHC) class I- and class II-restricted anti-tumour or anti-viral immunity. This review will focus on the development of mRNA-loaded DC-based immunotherapy vaccines. First, several published results concerning mRNA transfection efficiency in DC are compared. Next, an overview is given for several published studies describing CD8+ and CD4+ T-cell clone activation using RNA-loaded DC. These data show that RNA-loaded DC efficiently process and present antigenic epitopes. Next, published data from in vitro T-cell activation studies using RNA-loaded DC are summarized and provide evidence that RNA-loaded DC can efficiently stimulate in vitro primary and secondary immune responses. Finally, the summarized data provide evidence that RNA-loaded DC are a promising strategy for the development of future cancer vaccination strategies.
Figures
Fig. 1
Proposed model for the induction of immunity by type I dendritic cells (DC1).
Fig. 2
Comparison of EGFP mRNA electroporation and DNA electroporation in K562 cells. K562 cells were electroporated at 300 V and 150 _µ_F in a 4-mm standard electroporation cuvette with 20 _µ_g EGFP mRNA or 20 _µ_g EGFP DNA. In a time-course, the percentage of EGFP-positive cells was followed flowcytometrically by percentage (left side), as well as mean fluorescence intensity (MFI) of EGFP positive cells (right side).
Fig. 3
Antigen presentation by MAGE-A1 mRNA-electroporated dendritic cells. HLA-A1-positive immature Mo-DC were cultured with GM-CSF and IL-4 and transfected at day 6 of culture by electroporation with MAGE-A1 mRNA or with EGFP mRNA. HLA-A1-positive Mo-DC pulsed with a MAGE-A1 peptide, and HLA-A1-negative Mo-DC electroporated with MAGE-A1 mRNA or pulsed with MAGE-A1 peptide served as controls. Antigen-presenting cells (indicated on the left of the graph) were co-incubated with a HLA-A1-restricted MAGE-A1 specific CD8+ CTL clone to determine antigen loading eficiency, as reflected by IFN-γ production of the CTL clone. Results are shown as mean ± s.d.
Similar articles
- Precision cancer immunotherapy: optimizing dendritic cell-based strategies to induce tumor antigen-specific T-cell responses against individual patient tumors.
Osada T, Nagaoka K, Takahara M, Yang XY, Liu CX, Guo H, Roy Choudhury K, Hobeika A, Hartman Z, Morse MA, Lyerly HK. Osada T, et al. J Immunother. 2015 May;38(4):155-64. doi: 10.1097/CJI.0000000000000075. J Immunother. 2015. PMID: 25839441 - Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients.
Lesterhuis WJ, De Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A, Scharenborg NM, Van De Rakt M, Hesselink EJ, Figdor CG, Adema GJ, Punt CJ. Lesterhuis WJ, et al. Anticancer Res. 2010 Dec;30(12):5091-7. Anticancer Res. 2010. PMID: 21187495 Clinical Trial. - Dendritic cells transfected with interleukin-12 and tumor-associated antigen messenger RNA induce high avidity cytotoxic T cells.
Bontkes HJ, Kramer D, Ruizendaal JJ, Kueter EW, van Tendeloo VF, Meijer CJ, Hooijberg E. Bontkes HJ, et al. Gene Ther. 2007 Feb;14(4):366-75. doi: 10.1038/sj.gt.3302874. Epub 2006 Oct 12. Gene Ther. 2007. PMID: 17036057 - mRNA-based dendritic cell vaccines.
Benteyn D, Heirman C, Bonehill A, Thielemans K, Breckpot K. Benteyn D, et al. Expert Rev Vaccines. 2015 Feb;14(2):161-76. doi: 10.1586/14760584.2014.957684. Epub 2014 Sep 8. Expert Rev Vaccines. 2015. PMID: 25196947 Review. - mRNA Cancer Vaccines.
Fiedler K, Lazzaro S, Lutz J, Rauch S, Heidenreich R. Fiedler K, et al. Recent Results Cancer Res. 2016;209:61-85. doi: 10.1007/978-3-319-42934-2_5. Recent Results Cancer Res. 2016. PMID: 28101688 Review.
Cited by
- Bridging infectious disease vaccines with cancer immunotherapy: a role for targeted RNA based immunotherapeutics.
Sayour EJ, Sanchez-Perez L, Flores C, Mitchell DA. Sayour EJ, et al. J Immunother Cancer. 2015 Apr 21;3:13. doi: 10.1186/s40425-015-0058-0. eCollection 2015. J Immunother Cancer. 2015. PMID: 25901285 Free PMC article. - High-level antigen expression and sustained antigen presentation in dendritic cells nucleofected with wild-type viral mRNA but not DNA.
Melhem NM, Gleason SM, Liu XD, Barratt-Boyes SM. Melhem NM, et al. Clin Vaccine Immunol. 2008 Sep;15(9):1337-44. doi: 10.1128/CVI.00154-08. Epub 2008 Jul 30. Clin Vaccine Immunol. 2008. PMID: 18667638 Free PMC article. - CD133 mRNA-Loaded Dendritic Cell Vaccination Abrogates Glioma Stem Cell Propagation in Humanized Glioblastoma Mouse Model.
Do ASS, Amano T, Edwards LA, Zhang L, De Peralta-Venturina M, Yu JS. Do ASS, et al. Mol Ther Oncolytics. 2020 Jun 24;18:295-303. doi: 10.1016/j.omto.2020.06.019. eCollection 2020 Sep 25. Mol Ther Oncolytics. 2020. PMID: 32728617 Free PMC article. - Chronic Myelocytic Leukemia (CML) Patient-Derived Dendritic Cells Transfected with Autologous Total RNA Induces CML-Specific Cytotoxicity.
Yu L, Hu T, Zou T, Shi Q, Chen G. Yu L, et al. Indian J Hematol Blood Transfus. 2016 Dec;32(4):397-404. doi: 10.1007/s12288-016-0643-5. Epub 2016 Jan 21. Indian J Hematol Blood Transfus. 2016. PMID: 27812247 Free PMC article. - New developments in dendritic cell-based vaccinations: RNA translated into clinics.
Grünebach F, Müller MR, Brossart P. Grünebach F, et al. Cancer Immunol Immunother. 2005 Jun;54(6):517-25. doi: 10.1007/s00262-004-0605-x. Epub 2005 Jan 27. Cancer Immunol Immunother. 2005. PMID: 15838706 Free PMC article. Review.
References
- Van Tendeloo VFI, Van Broeckhoven C, Berneman ZN. Gene-based cancer vaccines: an ex vivo approach. Leukemia. 2001;15:545–58. - PubMed
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
- Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8 (+) regulatory T cells in vivo in humans. Blood. 2002;100:174–7. - PubMed
- Czerniecki BJ, Cohen PA, Faries M, Xu S, Roros JG, Bedrosian I. Diverse functional activity of CD83+ monocyte-derived dendritic cells and implications for cancer vaccines. Crit Rev Immunol. 2001;21:157–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials