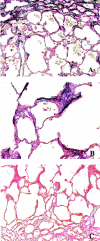Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia - PubMed (original) (raw)
Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia
Jack Gauldie et al. Am J Pathol. 2003 Dec.
Abstract
Bronchopulmonary dysplasia is a chronic lung disease of premature human infancy that shows pathological features comprising varying sized areas of interstitial fibrosis in association with distorted large alveolar spaces. We have previously shown that transfer of active transforming growth factor (TGF)-beta 1 (AdTGF beta 1(223/225)) genes by adenovirus vector to embryonic lungs results in inhibition of branching morphogenesis and primitive peripheral lung development, whereas transfer to adult lungs results in progressive interstitial fibrosis. Herein we show that transfer of TGF-beta1 to newborn rat pups results in patchy areas of interstitial fibrosis developing throughout a period of 28 days after transfer. These areas of fibrosis appear alongside areas of enlarged alveolar spaces similar to the prealveoli seen at birth, suggesting that postnatal lung development and alveolarization has been inhibited. In rats treated with AdTGF beta 1(223/225), enlarged alveolar spaces were evident by day 21, and by 28 days, the mean alveolar cord length was nearly twice that in control vector or untreated rats. Hydroxyproline measurements confirmed the presence of fibrosis. These data suggest that overexpression of TGF-beta 1 during the critical period of postnatal rat lung alveolarization gives rise to pathological, biochemical, and morphological changes consistent with those seen in human bronchopulmonary dysplasia, thus inferring a pathogenic role for TGF-beta in this disorder.
Figures
Figure 1.
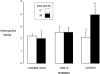
TGF-β1 levels in lung homogenate of 3-day-old rat, untreated, or 2 days after intranasal administration of 1 × 10 PFU AdDL70 or AdTGFβ1223/225. Levels of total TGF-β1 (white bars) and active TGF-β1 (black bars), as measured by enzyme-linked immunosorbent assay, are expressed as pg of TGF-β1 per mg of lung. Significant differences were seen between the TGF-β1 vector-treated group and the control groups for levels of total TGF-β1 (*, P < 0.02) and active TGF-β1 (#, P < 0.02; n = 3 to 5 per group. There were no significant differences between the control vector group and normal untreated animals.
Figure 2.
Eight-day-old neonatal rat lung (7 days after treatment). A, D: Control lung, untreated. B, E: Lung treated with AdDL70-3. C, F: Lung treated with AdTGFβ1223/225. A, B, and C were stained with H&E and D, E, and F were stained with Picrosirius Red. Original magnifications, ×200.
Figure 3.
Twenty-nine-day-old neonatal rat lung (28 days after treatment). A, B: Untreated. C, D: Treatment with control vector Ad dl70-3. A and C were stained with H&E and B and D were stained with Picrosirius Red. Lungs of both groups appear histologically normal. Original magnifications, ×100.
Figure 4.
Twenty-nine-day-old neonatal rat lung (28 days after treatment). Fibrotic response to AdTGFβ1223/225 vector. A and B were stained with EvG and C was stained with Picrosirius Red. Original magnifications: ×100 (A, C); ×200 (B).
Figure 5.
Hydroxyproline content of lungs of rats untreated or after intranasal administration of 1 × 10 PFU AdDL70 or AdTGFβ1223/225. Hydroxyproline content was analyzed 21 days (white bars) and 28 days (black bars) after treatment when the pups were 22 and 29 days old, respectively. TGF-β1 vector-treated group had significantly more hydroxyproline than the control vector group after 28 days, expressed as μg hydroxyproline per mg of wet lung tissue (P = 0.078, one-way analysis of variance; #, P < 0.05, _t_-test for TGF-β1 versus DL70) and was significantly different from day 21 TGF-β1-treated group (*, P < 0.05, _t_-test; n = 3 to 5 per group).
Figure 6.
Twenty-nine-day-old neonatal rat lung (28 days after treatment). A, C: Treatment with AdTGFβ1223/225. B, D: Untreated normal rat lung. A and B were stained with H&E and C and D were stained for α-SMA content. Original magnifications: ×100 (A, B); ×200 (C, D).
Figure 7.
Neonatal rat lungs develop spatially distinct pathologies of fibrosis and inhibited alveolarization 28 days after treatment with AdTGFβ1223/225. A was stained with H&E and shows the dense cellular infiltrate within the fibrotic area at the top. B is the same section stained with EvG (matrix stains dark purple) and shows the fibrotic area corresponds with the cellular infiltrate, yet is not associated with the underdeveloped alveoli. C was stained for α-SMA content. Original magnifications, ×50.
Figure 8.
Neonatal rat lungs 28 days after treatment with AdTGFβ1223/225. Alveolar tissue have elastin caps (dark stain), characteristic of developing alveoli. Stained with EvG. Original magnifications, ×400.
Similar articles
- Oxidative stress contributes to the induction and persistence of TGF-β1 induced pulmonary fibrosis.
Cui Y, Robertson J, Maharaj S, Waldhauser L, Niu J, Wang J, Farkas L, Kolb M, Gauldie J. Cui Y, et al. Int J Biochem Cell Biol. 2011 Aug;43(8):1122-33. doi: 10.1016/j.biocel.2011.04.005. Epub 2011 Apr 14. Int J Biochem Cell Biol. 2011. PMID: 21514399 - Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung.
Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Sime PJ, et al. J Clin Invest. 1997 Aug 15;100(4):768-76. doi: 10.1172/JCI119590. J Clin Invest. 1997. PMID: 9259574 Free PMC article. - Transforming growth factor-beta signaling across ages: from distorted lung development to chronic obstructive pulmonary disease.
Morty RE, Königshoff M, Eickelberg O. Morty RE, et al. Proc Am Thorac Soc. 2009 Dec 1;6(7):607-13. doi: 10.1513/pats.200908-087RM. Proc Am Thorac Soc. 2009. PMID: 19934357 Review. - Transgenic modeling of transforming growth factor-beta(1): role of apoptosis in fibrosis and alveolar remodeling.
Lee CG, Kang HR, Homer RJ, Chupp G, Elias JA. Lee CG, et al. Proc Am Thorac Soc. 2006 Jul;3(5):418-23. doi: 10.1513/pats.200602-017AW. Proc Am Thorac Soc. 2006. PMID: 16799085 Free PMC article. Review.
Cited by
- TGF-β controls alveolar type 1 epithelial cell plasticity and alveolar matrisome gene transcription in mice.
Callaway DA, Penkala IJ, Zhou S, Knowlton JJ, Cardenas-Diaz F, Babu A, Morley MP, Lopes M, Garcia BA, Morrisey EE. Callaway DA, et al. J Clin Invest. 2024 Jan 11;134(6):e172095. doi: 10.1172/JCI172095. J Clin Invest. 2024. PMID: 38488000 Free PMC article. - Stretch regulates alveologenesis and homeostasis via mesenchymal Gαq/11-mediated TGFβ2 activation.
Goodwin AT, John AE, Joseph C, Habgood A, Tatler AL, Susztak K, Palmer M, Offermanns S, Henderson NC, Jenkins RG. Goodwin AT, et al. Development. 2023 May 1;150(9):dev201046. doi: 10.1242/dev.201046. Epub 2023 May 12. Development. 2023. PMID: 37102682 Free PMC article. - The vascular phenotype of BPD: new basic science insights-new precision medicine approaches.
Durlak W, Thébaud B. Durlak W, et al. Pediatr Res. 2024 Oct;96(5):1162-1171. doi: 10.1038/s41390-022-02428-7. Epub 2022 Dec 22. Pediatr Res. 2024. PMID: 36550351 Review. - The Role of Insulin Receptor Substrate Proteins in Bronchopulmonary Dysplasia and Asthma: New Potential Perspectives.
Gorgisen G, Aydin M, Mboma O, Gökyildirim MY, Chao CM. Gorgisen G, et al. Int J Mol Sci. 2022 Sep 4;23(17):10113. doi: 10.3390/ijms231710113. Int J Mol Sci. 2022. PMID: 36077511 Free PMC article. Review. - TRAIL protects the immature lung from hyperoxic injury.
Shahzad T, Chao CM, Hadzic S, Behnke J, Biebach L, Böttcher-Friebertshäuser E, Wilhelm J, Hilgendorff A, Zimmer KP, Morty RE, Bellusci S, Ehrhardt H. Shahzad T, et al. Cell Death Dis. 2022 Jul 15;13(7):614. doi: 10.1038/s41419-022-05072-5. Cell Death Dis. 2022. PMID: 35840556 Free PMC article.
References
- Bancalari E: Changes in the pathogenesis and prevention of chronic lung disease of prematurity. Am J Perinatol 2001, 18:1-9 - PubMed
- Jobe AH, Ikegami M: Prevention of bronchopulmonary dysplasia. Curr Opin Pediatr 2001, 13:124-129 - PubMed
- Northway WH, Rosan RC, Porter DY: Pulmonary disease following respiratory therapy of hyaline membrane disease. Bronchopulmonary dysplasia N Engl J Med 1967, 276:357-368 - PubMed
- Jobe AH: The new BPD: an arrest of lung development. Pediatr Res 1999, 46:641-643 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous