Cognitive modulation of the cerebral processing of human oesophageal sensation using functional magnetic resonance imaging - PubMed (original) (raw)
Cognitive modulation of the cerebral processing of human oesophageal sensation using functional magnetic resonance imaging
L J Gregory et al. Gut. 2003 Dec.
Abstract
Background: While cortical processing of visceral sensation has been described, the role that cognitive factors play in modulating this processing remains unclear.
Aim: To investigate how selective and divided attention modulate the cerebral processing of oesophageal sensation.
Methods: In seven healthy volunteers (six males, mean age 33 years; ranging from 24 to 41 years old) from the general community, phasic visual and oesophageal (non-painful balloon distension) stimuli were presented simultaneously. During the selective attention task, subjects were instructed to press a button either to a change in frequency of oesophageal or visual stimuli. During a divided attention task, subjects received simultaneous visual and oesophageal stimuli and were instructed to press a button in response to a change in frequency of both stimuli.
Results: Selectively focussing attention on oesophageal stimuli activated the visceral sensory and cognitive neural networks (primary and secondary sensory cortices and anterior cingulate cortex respectively) while selective attention to visual stimuli primarily activated the visual cortex. When attention was divided between the two sensory modalities, more brain regions in the sensory and cognitive domains were utilised to process oesophageal stimuli in comparison to those employed to process visual stimuli (p=0.003).
Conclusion: Selective and divided attention to visceral stimuli recruits more neural resources in both the sensory and cognitive domains than attention to visual stimuli. We provide neurobiological evidence that demonstrates the biological importance placed on visceral sensations and demonstrate the influence of cognitive factors such as attention on the cerebral processing of visceral sensation.
Figures
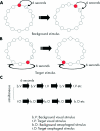
Figure 1
Schematic representation of the visual stimulus during background visual stimulation (A) and during target visual stimulation (B). During background visual stimulation, circles fill with colour in a clockwise direction at a rate of one every six seconds. As the next circle in the sequence is filled with colour, the previous circle empties. During target visual stimulation, there is a failure of a circle to fill with colour and the next circle following the empty circle fills with colour. When subjects detect the target visual stimulus, they indicate by pressing a button on a game pad. (C) Simplified schematic of protocol for selective and divided attention experiments. Note: The order of visual and oesophageal background and target stimuli are counterbalanced across all subjects for both selective and divided attention tasks. An interstimulus of six seconds occurs between each visual and oesophageal stimulus.
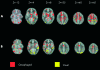
Figure 2
(A) Comparison of brain activation between selective attention to visual (yellow) and selective attention to oesophageal (red) stimuli. Images run inferior to superior (left to right). (B) Comparison of brain activation between divided attention to visual (yellow) and divided attention to oesophageal stimuli (red). Images run inferior to superior (top left to bottom right). Stereotactic (z) coordinate is shown across the top of the figure. Note: the comparisons of oesophageal v visual targets in both selective and divided attention experiments were generated from the product of the target stimuli of sensory modality 1 (for example, visual) subtracted from a standardised rest condition (that is generated from interstimulus intervals) being compared to the product of target stimuli of sensory modality 2 (for example, visceral) subtracted from a standardised rest condition.
Similar articles
- Attention and sensory interactions within the occipital cortex in the early blind: an fMRI study.
Weaver KE, Stevens AA. Weaver KE, et al. J Cogn Neurosci. 2007 Feb;19(2):315-30. doi: 10.1162/jocn.2007.19.2.315. J Cogn Neurosci. 2007. PMID: 17280519 - Functional neuroimaging of visceral sensation.
Aziz Q, Schnitzler A, Enck P. Aziz Q, et al. J Clin Neurophysiol. 2000 Nov;17(6):604-12. doi: 10.1097/00004691-200011000-00006. J Clin Neurophysiol. 2000. PMID: 11151978 Review. - Cortical processing of human somatic and visceral sensation.
Aziz Q, Thompson DG, Ng VW, Hamdy S, Sarkar S, Brammer MJ, Bullmore ET, Hobson A, Tracey I, Gregory L, Simmons A, Williams SC. Aziz Q, et al. J Neurosci. 2000 Apr 1;20(7):2657-63. doi: 10.1523/JNEUROSCI.20-07-02657.2000. J Neurosci. 2000. PMID: 10729346 Free PMC article. - The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation.
Phillips ML, Gregory LJ, Cullen S, Coen S, Ng V, Andrew C, Giampietro V, Bullmore E, Zelaya F, Amaro E, Thompson DG, Hobson AR, Williams SC, Brammer M, Aziz Q. Phillips ML, et al. Brain. 2003 Mar;126(Pt 3):669-84. doi: 10.1093/brain/awg065. Brain. 2003. PMID: 12566287 - From sensation to cognition.
Mesulam MM. Mesulam MM. Brain. 1998 Jun;121 ( Pt 6):1013-52. doi: 10.1093/brain/121.6.1013. Brain. 1998. PMID: 9648540 Review.
Cited by
- Cerebral Metabolic Analysis of Patients With Colorectal Cancer and Chronic Enteritis: Inquiry Into Gut-Brain Crosstalk.
Ma J, Wu JJ, Xing XX, Huo BB, Gao X, Ma ZZ, Li SS, Zheng MX, Hua XY, Xu JG. Ma J, et al. Front Neurosci. 2022 Feb 25;16:822891. doi: 10.3389/fnins.2022.822891. eCollection 2022. Front Neurosci. 2022. PMID: 35281497 Free PMC article. - On the Relationship of Interoceptive Accuracy and Attention: A Controlled Study With Depressed Inpatients and a Healthy Cohort.
Schultchen D, Schneider C, Berberich G, Zaudig M, Erle TM, Pollatos O. Schultchen D, et al. Front Psychol. 2021 Feb 1;11:597488. doi: 10.3389/fpsyg.2020.597488. eCollection 2020. Front Psychol. 2021. PMID: 33597903 Free PMC article. - Use of functional magnetic resonance imaging in patients with irritable bowel syndrome and functional dyspepsia.
Skrobisz K, Piotrowicz G, Drozdowska A, Markiet K, Sabisz A, Naumczyk P, Rydzewska G, Szurowska E. Skrobisz K, et al. Prz Gastroenterol. 2019;14(3):163-167. doi: 10.5114/pg.2019.88163. Epub 2019 Sep 27. Prz Gastroenterol. 2019. PMID: 31649785 Free PMC article. Review. - Differential functional brain network connectivity during visceral interoception as revealed by independent component analysis of fMRI TIME-series.
Jarrahi B, Mantini D, Balsters JH, Michels L, Kessler TM, Mehnert U, Kollias SS. Jarrahi B, et al. Hum Brain Mapp. 2015 Nov;36(11):4438-68. doi: 10.1002/hbm.22929. Epub 2015 Aug 7. Hum Brain Mapp. 2015. PMID: 26249369 Free PMC article. - Antibodies against gonadotropin-releasing hormone in patients with posterior laryngitis.
Pendleton H, Alm R, Nordin Fredrikson G, Ohlsson B. Pendleton H, et al. Drug Target Insights. 2013;7:1-8. doi: 10.4137/DTI.S10837. Epub 2013 Jan 28. Drug Target Insights. 2013. PMID: 23400339 Free PMC article.
References
- Hollerbach S, Fitzpatrick D, Shine G, et al. Cognitive evoked potentials to anticipated oesophageal stimulus in humans: quantitative assessment of the cognitive aspects of visceral perception. Neurogastroenterol Motil 1999;111:37–46. - PubMed
- Mayer EA, Gebhart G. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 1994;107:271–93. - PubMed
- Ness T, Gebhart G. Visceral pain: a review of experimental studies. Pain 1990;41:167–234. - PubMed
- Silverman D, Munakata JA, Ennes H, et al. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology 1997;112:64–72. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources