Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans - PubMed (original) (raw)
Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans
Sonja Potrebic et al. J Neurosci. 2003.
Abstract
Agonists at serotonin 1D (5-HT1D) receptors relieve migraine headache but are not clinically used as general analgesics. One possible explanation for this difference is that 5-HT1D receptors are preferentially expressed by cranial afferents of the trigeminal system. We compared the distribution of 5-HT1D receptor-immunoreactive (5-HT1D-IR) peripheral afferents within the trigeminal ganglion (TRG) and lumbar dorsal root ganglion (DRG) of the rat. We also examined the neurochemical identity of 5-HT1D-IR neurons with markers of primary afferent nociceptors, peripherin, isolectin B4, and substance P, and markers of myelinated afferents, N52 and SSEA3. We observed a striking similarity in the size, distribution, and neurochemical identity of 5-HT1D-IR neurons in TRG and lumbar DRG afferents. Furthermore, the vast majority of 5-HT1D-IR neurons are unmyelinated peptidergic afferents that distribute peripherally, including the dura, cornea, and the sciatic nerve. In the central projections of these afferents within the trigeminal nucleus caudalis and the spinal cord dorsal horn, 5-HT1D-IR fibers are concentrated in laminas I and outer II; a few axons penetrate to lamina V. At the ultrastructural level, 5-HT1D receptors in the spinal cord dorsal horn are localized exclusively within dense core vesicles of synaptic terminals. We observed scattered 5-HT1D-IR neurons in the nodose ganglia, and there was sparse terminal immunoreactivity in the solitary nucleus. The visceral efferents of the superior cervical ganglia did not contain 5-HT1D immunoreactivity. Our finding, that 5-HT1D receptors are distributed in nociceptors throughout the body, raises the possibility that triptans can regulate not only headache-associated pain but also nociceptive responses in extracranial tissues.
Figures
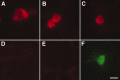
Figure 1.
Transfection control of the 5-HT1D antibody. HEK were transfected with the mouse cDNA for 5-HT1D (A-C), 5-HT1B (D), or 5-HT1F (E) receptors. F, GFP transfection control performed in parallel. There is strong immunoreactivity only when the 5-HT1D receptor is expressed. Scale bar, 20 μm.
Figure 2.
Dense and punctate 5-HT1D immunoreactivity in sensory ganglia. Low-power view shows 5-HT1D-IR neurons in both L5 DRG (A) and TRG (B). Examples of the two patterns of neuronal 5-HT1D immunoreactivity, dense and punctate, are seen in DRG (C) and TRG (D).
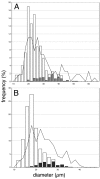
Figure 3.
Stacked histogram of the distribution of 5-HT1D-IR neuron diameters in the DRG (A) and TRG (B). The relative contributions of dense (open) and punctate (filled) patterns of immunostaining to the distribution are indicated. The overlaid line graph shows the frequency distribution of cell diameters in the entire DRG or TRG population (both labeled and unlabeled).
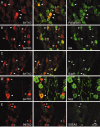
Figure 4.
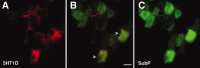
5-HT1D receptors predominate in nociceptors. Immunofluorescent labeling of ganglion cells in the lumbar DRG (A, D, E) and TRG (B, C). Staining of 5-HT1D receptors is shown in the left panels, and colocalization with different markers is shown on the right: peripherin (A), IB4 binding (B), substance P (C), N52 (D), and SSEA3 (E). Middle panels show merged images. Arrowheads, Double-labeled neurons. Arrows, Single-labeled 5-HT1D-IR neurons
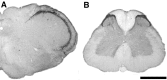
Figure 5.
5-HT1D expression in the trigeminal nucleus caudalis and lumbar spinal cord of the rat. These 50 μm sections of the caudal medulla and lumbar spinal cord were stained with 5-HT1D antibody and HRP detected by reaction with DAB. A, There is dense terminal immunoreactivity in the trigeminal nucleus and much lighter terminal labeling in the solitary nucleus. B, There is also dense terminal staining in the superficial laminas of the dorsal horn. Scale bar, 1 mm.
Figure 6.
5-HT1D colocalizes with a subpopulation of peptidergic visceral afferents in the nodose ganglia. A, 5-HT1D. C, SP. B, Merged image shows that a subset of SP visceral afferents are double labeled with 5-HT1D receptor (arrowheads). Scale bar, 20 μm.
Figure 7.
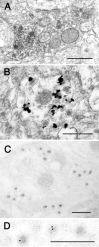
Electron microscopic immunolocalization of 5-HT1D in DCVs of terminals in lamina I. A, These terminals, immunolabeled with HRP and DAB, show a flocculent 5-HT1D immunoreaction product that is most strongly concentrated over DCVs. B, Immunolabeling with a silver intensified preembedding method also shows label only over the DCVs. C, D, Double-label postembedding immunogold reveals colocalization of 5-HT1D (15 nm gold particles) and SP (6 nm gold particles) in the same DCVs. This single large axon terminal has four peripheral groups of DCVs in the plane of section, each of which is immunoreactive for both antigens. D shows the immunolabeling from a different terminal at higher magnification. Scale bars, 0.5 μm.
Figure 8.
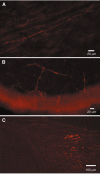
5-HT1D-IR fibers in peripheral tissue. Immunofluorescent labeling of 5-HT1D-IR fibers in dura (A), cornea (B), and ligated sciatic nerve (C). In C, there is buildup of label proximal to the ligature (right) with much reduced label distally.
Similar articles
- Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats.
Ma QP, Hill R, Sirinathsinghji D. Ma QP, et al. Eur J Neurosci. 2001 Jun;13(11):2099-104. doi: 10.1046/j.0953-816x.2001.01586.x. Eur J Neurosci. 2001. PMID: 11422450 - Tissue injury regulates serotonin 1D receptor expression: implications for the control of migraine and inflammatory pain.
Ahn AH, Basbaum AI. Ahn AH, et al. J Neurosci. 2006 Aug 9;26(32):8332-8. doi: 10.1523/JNEUROSCI.1989-06.2006. J Neurosci. 2006. PMID: 16899728 Free PMC article. - Transgenic Mouse Models for the Tracing of “Pain” Pathways.
Basbaum AI, Bráz JM. Basbaum AI, et al. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 7. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 7. PMID: 21882471 Free Books & Documents. Review. - The science of migraine.
Burstein R, Jakubowski M, Rauch SD. Burstein R, et al. J Vestib Res. 2011;21(6):305-14. doi: 10.3233/VES-2012-0433. J Vestib Res. 2011. PMID: 22348935 Free PMC article. Review.
Cited by
- Electrophysiological properties and chemosensitivity of acutely dissociated trigeminal somata innervating the cornea.
Veiga Moreira TH, Gover TD, Weinreich D. Veiga Moreira TH, et al. Neuroscience. 2007 Sep 7;148(3):766-74. doi: 10.1016/j.neuroscience.2007.03.056. Epub 2007 Aug 15. Neuroscience. 2007. PMID: 17706884 Free PMC article. - The perception and endogenous modulation of pain.
Ossipov MH. Ossipov MH. Scientifica (Cairo). 2012;2012:561761. doi: 10.6064/2012/561761. Epub 2012 Dec 25. Scientifica (Cairo). 2012. PMID: 24278716 Free PMC article. Review. - Sensory innervation of the calvarial bones of the mouse.
Kosaras B, Jakubowski M, Kainz V, Burstein R. Kosaras B, et al. J Comp Neurol. 2009 Jul 20;515(3):331-48. doi: 10.1002/cne.22049. J Comp Neurol. 2009. PMID: 19425099 Free PMC article. - The protective effect of chemical and natural compounds against vincristine-induced peripheral neuropathy (VIPN).
Khodaei M, Mehri S, Pour SR, Mahdavi S, Yarmohammadi F, Hayes AW, Karimi G. Khodaei M, et al. Naunyn Schmiedebergs Arch Pharmacol. 2022 Aug;395(8):907-919. doi: 10.1007/s00210-022-02254-y. Epub 2022 May 14. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 35562512 Review. - Cutaneous allodynia as predictor for treatment response in chronic migraine: a cohort study.
Pijpers JA, Kies DA, van Zwet EW, de Boer I, Terwindt GM. Pijpers JA, et al. J Headache Pain. 2023 Aug 30;24(1):118. doi: 10.1186/s10194-023-01651-9. J Headache Pain. 2023. PMID: 37644420 Free PMC article. Clinical Trial.
References
- al Balawi S, Tariq M, Feinmann C ( 1996) A double-blind, placebo-controlled, crossover, study to evaluate the efficacy of subcutaneous sumatriptan in the treatment of atypical facial pain. Int J Neurosci 86: 301-309. - PubMed
- Amlaiky N, Ramboz S, Boschert U, Plassat JL, Hen R ( 1992) Isolation of a mouse “5HT1E-like” serotonin receptor expressed predominantly in hippocampus. J Biol Chem 267: 19761-19764. - PubMed
- Antonaci F, Pareja JA, Caminero AB, Sjaastad O ( 1998) Chronic paroxysmal hemicrania and hemicrania continua: lack of efficacy of sumatriptan. Headache 38: 197-200. - PubMed
- Bonaventure P, Voorn P, Luyten WH, Leysen JE ( 1998) 5HT1B and 5HT1D receptor mRNA differential co-localization with peptide mRNA in the guinea pig trigeminal ganglion. NeuroReport 9: 641-645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 NS014627/NS/NINDS NIH HHS/United States
- NS21445/NS/NINDS NIH HHS/United States
- P50 NS021445/NS/NINDS NIH HHS/United States
- NS14627/NS/NINDS NIH HHS/United States
- NS02237/NS/NINDS NIH HHS/United States
- R01 NS014627/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases