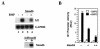Smad6 recruits transcription corepressor CtBP to repress bone morphogenetic protein-induced transcription - PubMed (original) (raw)
Smad6 recruits transcription corepressor CtBP to repress bone morphogenetic protein-induced transcription
Xia Lin et al. Mol Cell Biol. 2003 Dec.
Abstract
Smad6 and Smad7 are inhibitory Smads induced by transforming growth factor beta-Smad signal transduction pathways in a negative-feedback mechanism. Previously it has been thought that inhibitory Smads bind to the type I receptor and block the phosphorylation of receptor-activated Smads, thereby inhibiting the initiation of Smad signaling. Conversely, few studies have suggested the possible nuclear functions of inhibitory Smads. Here, we present compelling evidence demonstrating that Smad6 repressed bone morphogenetic protein-induced Id1 transcription through recruiting transcriptional corepressor C-terminal binding protein (CtBP). A consensus CtBP-binding motif, PLDLS, was identified in the linker region of Smad6. Our findings show that mutation in the motif abolished the Smad6 binding to CtBP and subsequently its repressor activity of transcription. We conclude that the nuclear functions and physical interaction of Smad6 and CtBP provide a novel mechanism for the transcriptional regulation by inhibitory Smads.
Figures
FIG. 1.
Smad6 inhibits BMP-induced Id1 transcription. (A) Smad6 inhibits BMP2-induced Id1 mRNA accumulation. Exponentially growing mouse embryonic P19 cells were infected with adenovirus Smad6, and then treated with 25 ng of BMP2 per ml, as indicated. Id1 mRNA was detected by Northern hybridization. Equal levels of RNA were loaded per lane. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (B) Smad6 inhibits BMP-induced activation of the Id1 promoter in P19 cells. Cells were transfected with the Id1-luc reporter plasmid and, at 36 h after transfection, treated with BMP for 12 h; luciferase values were measured. RLU, relative light units.
FIG. 2.
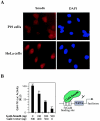
Smad6 has repressor activity. (A) Smad6 is localized in the nucleus and cytoplasm. Endogenous Smad6 was detected using anti-Smad6 staining followed by FITC-conjugated secondary antibody and visualized under a fluorescence microscope. DAPI indicates nuclear staining. (B) Smad6 has transcriptional repressor activity. P19 cells were cotransfected with Gal4-Smad6 and the luciferase reporter plasmid pGal4-TK-luc. The basal transactivation of the Gal4-TK-luc reporter is scored as 100 in the absence of Gal4-Smad6. Note that increasing levels of Gal4-Smad6 have an inhibitory activity on the Gal4-binding promoter. RLU, relative light units.
FIG. 3.
Nucleus-targeted Smad6 retains the ability to repress the BMP-induced Id1 response. (A) Schematic diagram of the structure of Smad6 and mutant versions of it. NLS-Smad6 contains an N-terminally fused NLS from SV40 large T antigen. (B) NLS-Smad6 is localized exclusively in the nucleus. NLS-Smad6 was transfected into P19 cells, and its expression was detected using anti-Smad6 staining followed by FITC-conjugated secondary antibody.DAPI indicates nuclear staining. (C) NLS-Smad6 does not bind to ALK3. 293T cells were transfected with HA-tagged ALK3 and Smad6 constructs. Cell lysates were immunoprecipitated (IP) with anti-HA antibodies (12CA5) and then immunoblotted (IB) with an anti-Smad6 polyclonal antibody to detect receptor-bound Smad6 (upper panel). Whole-cell lysates (WCL) were also directly immunoblotted with anti-HA or anti-Smad6 antibodies to demonstrate the expression of ALK3 and Smad6 (bottom panels). (D) NLS-Smad6 is capable of repressing the Id1 promoter. NLS-Smad6 was transfected into P19 cells together with Id1-luciferase reporter. BMP treatment and luciferase measurement were carried out as described in the legend to Fig. 1B. (E) Smad6NL and Smad6C synergize to repress the Id1 promoter. Various Smad6 constructs were transfected into P19 cells together with Id1-luciferase reporter. BMP treatment and luciferase measurement were carried out as described in the legend to Fig. 1B. Note that Smad6NL alone retains ability to repress the Id1 promoter. RLU, relative light units.
FIG. 4.
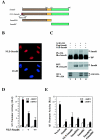
Smad6 interacts with the transcription corepressor CtBP in vivo. (A) Smad6 coimmunoprecipitates with CtBP. 293T cells were transfected with HA-tagged Smad6 and myc-tagged CtBP. Cell lysates were immunoprecipitated (IP) with anti-HA antibodies (12CA5 or HA1.1)and then immunoblotted (IB) with an anti-Myc polyclonal antibody to detect Smad6-bound CtBP (upper panel) and anti-HA to determine the level of immunoprecipitated Smad6 (bottom). Whole-cell lysates (WCL) were also directly immunoblotted with anti-Myc or anti-HA antibodies to demonstrate the expression of transfected CtBP and Smad6 (lanes 7 and 8). (B) Smad6 interaction with CtBP is specific. Immunoprecipitation-Western analysis was conducted as for panel A, except that anti-Flag and anti-HA antibodies were used for IP and IB, respectively. (C) Endogenous interaction between Smad6 and CtBP. The IP-Western blot procedure was carried out similarly to that in panel A, with the antibodies indicated. (D) Smad6 is colocalized with CtBP. Immunofluorescence was performed as in Fig. 2A.
FIG. 5.
The Smad6 NL domain mediates its interaction with CtBP. (A) Schematic diagram of Smad6 constructs. Deletion mutants of Smad6 and Smad6-Smad7 chimera were shown by different shadings: dark gray, MH1; light gray, linker; medium gray, MH2. Their interaction with CtBP from immunoprecipitation experiments (B; see also Fig. 6C) is summarized. (B) Mapping of the CtBP-interacting domain on Smad6. 293T cells were transfected with HA-tagged Smad constructs and myc-tagged CtBP. Smad-bound CtBP was detected by anti-HA immunoprecipitation (IP) coupled with anti-Myc immunoblotting (IB) (upper panel). The levels of transfected protein in whole-cell lysates (WCL) are shown (bottom panels).
FIG. 6.
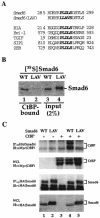
The PLDLS motif is essential for Smad6 interaction with CtBP. (A) Alignment of PLDLS motifs. The PLDLS motif of Smad6 and the mutated version are shown together with other CtBP-binding proteins. (B) The PLDLS→LLAVS mutation abrogates the binding of Smad6 to CtBP. Equal amounts (1 μg) of GST-CtBP fusion protein on glutathione-Sepharose beads were incubated with 35S-labeled Smad6 in vitro. After extensive washing, CtBP-Smad6 complex was resolved by SDS-PAGE and CtBP-bound Smad6 was visualized by autoradiography. WT, wild-type Smad6; LAV, Smad6(LAV) mutant. (C) The PLDLS mutant of Smad6 fails to interact with CtBP in vivo. 293T cells were transfected with HA-tagged Smad6 and myc-tagged CtBP. Smad6-bound CtBP was detected by anti-HA IP coupled with anti-Myc IB (upper panel). The levels of transfected protein in whole-cell lysates are shown. WT, wild-type Smad6; LAV, Smad6(LAV) mutant.
FIG. 7.
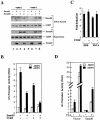
Smad6 and CtBP are recruited to the Id1 promoter, and they cooperate to repress the BMP-induced Id1 transcription. (A) Smad6 and CtBP coexist on the Id1 promoter. 293T cells were transfected with expression plasmids for Smad1 and Smad6 and treated with BMP2 for 4 h as indicated. (B) Smad6 cooperates with CtBP to inhibit BMP-dependent Id1 induction. P19 cells were transfected with the indicated expression plasmids. BMP2 treatment and the Id1-luciferase assay were done as in Fig. 1B. (C) Overexpression of CtBP has no effects on TGF-β-induced promoter activity. TGF-β-responsive HaCaT cells were transfected with SBE-luc or PAI1-luc, with or without CtBP, and treated with 5 ng of TGF-β per ml for 24 h. The _y_-axis value represents the induction by TGF-β, i.e., the ratio of Id1 promoter activity in the presence of TGF-β to that in the absence of TGF-β. (D) TSA inhibits the ability of Smad6 to repress BMP-induced Id1 induction. HeLa cells were transfected with the indicated Smad6 expression plasmids and treated with 10 ng of TSA per ml for 18 h. BMP2 treatment and the Id1-luciferase assay were done as in Fig. 1B. RLU, relative light units.
FIG. 8.
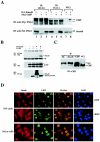
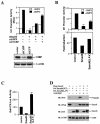
CtBP binding is required for maximal inhibitory function of Smad6 in BMP-induced Id1 transcription. (A) siRNA of CtBP blocks Smad6-mediatd repression on the BMP-dependent Id1 promoter activity. HeLa cells were transfected with the indicated siRNA expression plasmids. BMP2 treatment and the Id1-luciferase assay were done as in Fig. 1B. The bottom panel shows the expression levels of CtBP knocked down by siCtBP but not by siGFP. (B) Smad6 mutant defective in CtBP binding has reduced activity to inhibit Id1 induction. P19 cells were transfected with the indicated expression plasmids. BMP2 treatment and the Id1-luciferase assay were done as in Fig. 1B. In the top panel, the y axis indicates relative light units (RLU) with or without BMP2; in bottom panel, the y axis represents BMP-mediated induction, i.e., the ratio of Id1 promoter activity in the presence of BMP2 to that in the absence of BMP2. (C) The Smad6 mutant defective in CtBP binding has lost its repressor activity. P19 cells were transfected and the Gal4-TK-luc reporter was scored as described in the legend to Fig. 2B. Smad6(LAV), the PLDLS mutant of Smad6 in Gal4 vector. Note that Gal4-Smad6(LAV) was converted from a repressor to an activator. (D) Loss of CtBP binding has no effect on the ability of Smad6 to inhibit receptor-mediated Smad1 phosphorylation. P19 cells were transfected with indicated expression plasmids, and after 48 h the cell lysates were subjected to Western blot analysis using the indicated antibodies. αPS1, antibody against phospho-Smad1.
FIG. 9.
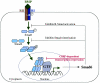
Working model for Smad6 functions in BMP signaling. The BMP signaling pathway is illustrated with points of Smad6 action. Smad6, an immediate-early gene product of BMP signaling, inhibits R-Smad activation and R-Smad-Smad4 heteromerization and directly represses transcription in the nucleus. RI, BMP type I receptor; RII, BMP type II receptor; GTF, general transcription factors such as TATA-binding proteins and associated factors.
Similar articles
- Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads.
Itoh F, Asao H, Sugamura K, Heldin CH, ten Dijke P, Itoh S. Itoh F, et al. EMBO J. 2001 Aug 1;20(15):4132-42. doi: 10.1093/emboj/20.15.4132. EMBO J. 2001. PMID: 11483516 Free PMC article. - Smad6 as a transcriptional corepressor.
Bai S, Shi X, Yang X, Cao X. Bai S, et al. J Biol Chem. 2000 Mar 24;275(12):8267-70. doi: 10.1074/jbc.275.12.8267. J Biol Chem. 2000. PMID: 10722652 - TGF-beta signaling by Smad proteins.
Miyazono K. Miyazono K. Cytokine Growth Factor Rev. 2000 Mar-Jun;11(1-2):15-22. doi: 10.1016/s1359-6101(99)00025-8. Cytokine Growth Factor Rev. 2000. PMID: 10708949 Review. - The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling.
Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K. Hanyu A, et al. J Cell Biol. 2001 Dec 10;155(6):1017-27. doi: 10.1083/jcb.200106023. Epub 2001 Dec 10. J Cell Biol. 2001. PMID: 11739411 Free PMC article. - Remarkable versatility of Smad proteins in the nucleus of transforming growth factor-beta activated cells.
Verschueren K, Huylebroeck D. Verschueren K, et al. Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):187-99. doi: 10.1016/s1359-6101(99)00012-x. Cytokine Growth Factor Rev. 1999. PMID: 10647776 Review.
Cited by
- Arginine methylation of R81 in Smad6 confines BMP-induced Smad1 signaling.
Wu J, Chen X, Sehgal P, Zhang T, Jackson-Weaver O, Gou Y, Bautch V, Frenkel B, Sun H, Xu J. Wu J, et al. J Biol Chem. 2021 Jan-Jun;296:100496. doi: 10.1016/j.jbc.2021.100496. Epub 2021 Mar 3. J Biol Chem. 2021. PMID: 33667543 Free PMC article. - C-Terminal Binding Protein: A Molecular Link between Metabolic Imbalance and Epigenetic Regulation in Breast Cancer.
Byun JS, Gardner K. Byun JS, et al. Int J Cell Biol. 2013;2013:647975. doi: 10.1155/2013/647975. Epub 2013 May 20. Int J Cell Biol. 2013. PMID: 23762064 Free PMC article. - TGFβ Signaling in Tumor Initiation, Epithelial-to-Mesenchymal Transition, and Metastasis.
Papageorgis P. Papageorgis P. J Oncol. 2015;2015:587193. doi: 10.1155/2015/587193. Epub 2015 Mar 25. J Oncol. 2015. PMID: 25883652 Free PMC article. Review. - Smad7 protein induces interferon regulatory factor 1-dependent transcriptional activation of caspase 8 to restore tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis.
Hong S, Kim HY, Kim J, Ha HT, Kim YM, Bae E, Kim TH, Lee KC, Kim SJ. Hong S, et al. J Biol Chem. 2013 Feb 1;288(5):3560-70. doi: 10.1074/jbc.M112.400408. Epub 2012 Dec 19. J Biol Chem. 2013. PMID: 23255602 Free PMC article. - PRMT5 Enables Robust STAT3 Activation via Arginine Symmetric Dimethylation of SMAD7.
Cai C, Gu S, Yu Y, Zhu Y, Zhang H, Yuan B, Shen L, Yang B, Feng XH. Cai C, et al. Adv Sci (Weinh). 2021 Feb 24;8(10):2003047. doi: 10.1002/advs.202003047. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026434 Free PMC article.
References
- Afrakhte, M., A. Moren, S. Jossan, S. Itoh, K. Sampath, B. Westermark, C. H. Heldin, N. E. Heldin, and P. ten Dijke. 1998. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-β family members. Biochem. Biophys. Res. Commun. 249:505-511. - PubMed
- Akiyoshi, S., H. Inoue, J. Hanai, K. Kusanagi, N. Nemoto, K. Miyazono, and M. Kawabata. 1999. c-Ski acts as a transcriptional co-repressor in transforming growth factor-β signaling through interaction with Smads. J. Biol. Chem. 274:35269-35277. - PubMed
- Bai, S., and X. Cao. 2002. A nuclear antagonistic mechanism of inhibitory Smads in transforming growth factor-β signaling. J. Biol. Chem. 277:4176-4182. - PubMed
- Bai, S., X. Shi, X. Yang, and X. Cao. 2000. Smad6 as a transcriptional corepressor. J. Biol. Chem. 275:8267-8270. - PubMed
- Blobe, G. C., W. P. Schiemann, and H. F. Lodish. 2000. Role of transforming growth factor β in human disease. N. Engl. J. Med. 342:1350-1358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM 63773/GM/NIGMS NIH HHS/United States
- F32 GM 70690/GM/NIGMS NIH HHS/United States
- R01 CA095731/CA/NCI NIH HHS/United States
- F32 GM070690/GM/NIGMS NIH HHS/United States
- R01 GM053874/GM/NIGMS NIH HHS/United States
- R01 CA 95731/CA/NCI NIH HHS/United States
- R01 GM063773/GM/NIGMS NIH HHS/United States
- R01 GM 53874/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources