Cancerous stem cells can arise from pediatric brain tumors - PubMed (original) (raw)
Cancerous stem cells can arise from pediatric brain tumors
Houman D Hemmati et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Pediatric brain tumors are significant causes of morbidity and mortality. It has been hypothesized that they derive from self-renewing multipotent neural stem cells. Here, we tested whether different pediatric brain tumors, including medulloblastomas and gliomas, contain cells with properties similar to neural stem cells. We find that tumor-derived progenitors form neurospheres that can be passaged at clonal density and are able to self-renew. Under conditions promoting differentiation, individual cells are multipotent, giving rise to both neurons and glia, in proportions that reflect the tumor of origin. Unlike normal neural stem cells, however, tumor-derived progenitors have an unusual capacity to proliferate and sometimes differentiate into abnormal cells with multiple differentiation markers. Gene expression analysis reveals that both whole tumors and tumor-derived neurospheres express many genes characteristic of neural and other stem cells, including CD133, Sox2, musashi-1, bmi-1, maternal embryonic leucine zipper kinase, and phosphoserine phosphatase, with variation from tumor to tumor. After grafting to neonatal rat brains, tumor-derived neurosphere cells migrate, produce neurons and glia, and continue to proliferate for more than 4 weeks. The results show that pediatric brain tumors contain neural stem-like cells with altered characteristics that may contribute to tumorigenesis. This finding may have important implications for treatment by means of specific targeting of stem-like cells within brain tumors.
Figures
Fig. 1.
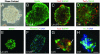
Tumor-derived progenitors form neurospheres in culture that give rise to both neuronal and glial cells. Neurospheres from one tumor, BT1, were cultured at medium (A–D) and clonal (E–H) densities. (A) A typical primary neurosphere. (B and C) Undifferentiated primary neurospheres expressed high levels of nestin protein (B, green) and low levels of β-III-tubulin (C, red) and GFAP (C, green). (D) Expression of β-III-tubulin and GFAP, after 7 days of differentiation on substrate. (E) Nestin expression in undifferentiated clonal neurosphere cells. (F) Musashi-1 (green) expression in undifferentiated clonal neurospheres. (G) β-III-tubulin (red) and GFAP (green) expression in a differentiated clonal neurosphere. Some cells (arrows) expressed both markers. (H) Hu (green) expression in a differentiated neurosphere. Some nuclei were counterstained with DAPI (F and H, blue). (Scale bar in H = 30 μm in A, G, and H, 60 μm in B–F.)
Fig. 2.
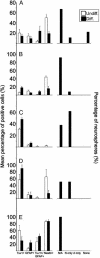
Neurospheres derived from multiple types of tumors give rise to cells expressing neuronal and glial markers in various proportions. (Left) Average count of cells expressing nestin, TuJ1 alone, GFAP alone, or both markers in clonal neurospheres (NS) from BT1–5 (A–E) before (white) and after (black) differentiation. (Right) Fates of clonal neurospheres (NS) after differentiation. Markers used are TuJ1 for neurons (N) and GFAP for astrocytes (A).
Fig. 3.
Immunohistochemical characteristics of original tumor samples. Paraffin-embedded sections were labeled with antibodies to nestin (green; A–D) or TuJ1 (red; E–H) to recognize neurons or GFAP (green; E–H) to recognize glia. Area denoted by asterisk in C and F delineates normal brain tissue adjacent to the tumor. (Scale bar in H = 60 μm in A–H.)
Fig. 4.
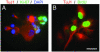
Tumor-derived neurospheres give rise to neurons that proliferate aberrantly. (A) Clonal neurosphere derived from the BT4 tumor, double-labeled for β-III-tubulin (red) and Ki-67 (green). Nuclei were counterstained with DAPI (blue). (B) Double-label for BrdUrd (green), visualized after a 14-h pulse and TuJ1 (red). (Scale bar in B = 15 μm in A and B.)
Similar articles
- Somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem cell-derived progenitors.
Kania G, Corbeil D, Fuchs J, Tarasov KV, Blyszczuk P, Huttner WB, Boheler KR, Wobus AM. Kania G, et al. Stem Cells. 2005 Jun-Jul;23(6):791-804. doi: 10.1634/stemcells.2004-0232. Stem Cells. 2005. PMID: 15917475 - Gliosarcoma stem cells undergo glial and mesenchymal differentiation in vivo.
deCarvalho AC, Nelson K, Lemke N, Lehman NL, Arbab AS, Kalkanis S, Mikkelsen T. deCarvalho AC, et al. Stem Cells. 2010 Feb;28(2):181-90. doi: 10.1002/stem.264. Stem Cells. 2010. PMID: 19937755 Free PMC article. - Regulation of glioblastoma stem cells by retinoic acid: role for Notch pathway inhibition.
Ying M, Wang S, Sang Y, Sun P, Lal B, Goodwin CR, Guerrero-Cazares H, Quinones-Hinojosa A, Laterra J, Xia S. Ying M, et al. Oncogene. 2011 Aug 4;30(31):3454-67. doi: 10.1038/onc.2011.58. Epub 2011 Mar 7. Oncogene. 2011. PMID: 21383690 Free PMC article. - On the origin and growth of gliomas.
Schiffer D, Annovazzi L, Caldera V, Mellai M. Schiffer D, et al. Anticancer Res. 2010 Jun;30(6):1977-98. Anticancer Res. 2010. PMID: 20651342 Review. - [Cancer stem cells in pediatric brain tumors].
Nakano I, Hemmati HD, Kornblum HI. Nakano I, et al. No Shinkei Geka. 2004 Aug;32(8):827-34. No Shinkei Geka. 2004. PMID: 15478649 Review. Japanese.
Cited by
- Isolation, cultivation and identification of brain glioma stem cells by magnetic bead sorting.
Zhou X, Zheng C, Shi Q, Li X, Shen Z, Yu R. Zhou X, et al. Neural Regen Res. 2012 May 5;7(13):985-92. doi: 10.3969/j.issn.1673-5374.2012.13.004. Neural Regen Res. 2012. PMID: 25722686 Free PMC article. - Unique genome-wide map of TCF4 and STAT3 targets using ChIP-seq reveals their association with new molecular subtypes of glioblastoma.
Zhang JX, Zhang J, Yan W, Wang YY, Han L, Yue X, Liu N, You YP, Jiang T, Pu PY, Kang CS. Zhang JX, et al. Neuro Oncol. 2013 Mar;15(3):279-89. doi: 10.1093/neuonc/nos306. Epub 2013 Jan 7. Neuro Oncol. 2013. PMID: 23295773 Free PMC article. - Oncolytic measles virus efficacy in murine xenograft models of atypical teratoid rhabdoid tumors.
Studebaker AW, Hutzen B, Pierson CR, Shaffer TA, Raffel C, Jackson EM. Studebaker AW, et al. Neuro Oncol. 2015 Dec;17(12):1568-77. doi: 10.1093/neuonc/nov058. Epub 2015 Apr 2. Neuro Oncol. 2015. PMID: 25838138 Free PMC article. - Wnt activation promotes neuronal differentiation of glioblastoma.
Rampazzo E, Persano L, Pistollato F, Moro E, Frasson C, Porazzi P, Della Puppa A, Bresolin S, Battilana G, Indraccolo S, Te Kronnie G, Argenton F, Tiso N, Basso G. Rampazzo E, et al. Cell Death Dis. 2013 Feb 21;4(2):e500. doi: 10.1038/cddis.2013.32. Cell Death Dis. 2013. PMID: 23429286 Free PMC article. - Exploiting molecular biology for diagnosis and targeted management of pediatric low-grade gliomas.
Garcia MA, Solomon DA, Haas-Kogan DA. Garcia MA, et al. Future Oncol. 2016 Jun;12(12):1493-506. doi: 10.2217/fon-2016-0039. Epub 2016 Apr 13. Future Oncol. 2016. PMID: 27072750 Free PMC article. Review.
References
- Smith, M. A., Freidlin, B., Ries, L. A. & Simon, R. (1998) J. Natl. Cancer Inst. 90, 1269-1277. - PubMed
- Sklar, C. A. (2002) J. Pediatr. Endocrinol. Metab. 15, Suppl. 2, 669-673. - PubMed
- MacDonald, T. J., Rood, B. R., Santi, M. R., Vezina, G., Bingaman, K., Cogen, P. H. & Packer, R. J. (2003) Oncologist 8, 174-186. - PubMed
- Brustle, O. & McKay, R. D. (1995) J. Neurooncol. 24, 57-59. - PubMed
- Holland, E. C. (2000) Toxicol. Pathol. 28, 171-177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM08042/GM/NIGMS NIH HHS/United States
- R01 NS042287/NS/NINDS NIH HHS/United States
- T32 GM008042/GM/NIGMS NIH HHS/United States
- R01 MH065756/MH/NIMH NIH HHS/United States
- MH65756/MH/NIMH NIH HHS/United States
- NS42287/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials