Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus - PubMed (original) (raw)
. 2003 Nov 27;426(6965):450-4.
doi: 10.1038/nature02145.
Michael J Moore, Natalya Vasilieva, Jianhua Sui, Swee Kee Wong, Michael A Berne, Mohan Somasundaran, John L Sullivan, Katherine Luzuriaga, Thomas C Greenough, Hyeryun Choe, Michael Farzan
Affiliations
- PMID: 14647384
- PMCID: PMC7095016
- DOI: 10.1038/nature02145
Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus
Wenhui Li et al. Nature. 2003.
Abstract
Spike (S) proteins of coronaviruses, including the coronavirus that causes severe acute respiratory syndrome (SARS), associate with cellular receptors to mediate infection of their target cells. Here we identify a metallopeptidase, angiotensin-converting enzyme 2 (ACE2), isolated from SARS coronavirus (SARS-CoV)-permissive Vero E6 cells, that efficiently binds the S1 domain of the SARS-CoV S protein. We found that a soluble form of ACE2, but not of the related enzyme ACE1, blocked association of the S1 domain with Vero E6 cells. 293T cells transfected with ACE2, but not those transfected with human immunodeficiency virus-1 receptors, formed multinucleated syncytia with cells expressing S protein. Furthermore, SARS-CoV replicated efficiently on ACE2-transfected but not mock-transfected 293T cells. Finally, anti-ACE2 but not anti-ACE1 antibody blocked viral replication on Vero E6 cells. Together our data indicate that ACE2 is a functional receptor for SARS-CoV.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
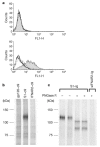
Figure 1. A 110 kDa protein associates with the S1 domain of SARS-CoV S protein.
a, A fusion protein of the S1 domain (S1–Ig, shaded area), or of the first 327 residues of that domain (S1(327)–Ig, dotted line) with the Fc domain of human IgG1, or culture medium alone (thick line) was incubated with 293T (top panel) or Vero E6 (bottom panel) cells. Binding of fusion proteins to cells was measured by flow cytometry using a FITC-labelled anti-human IgG secondary antibody. b, Metabolically labelled Vero E6 cell lysates were immunoprecipitated with HIV-1 gp120, SARS-CoV S1 domain, or the ectodomain of human IFNAR2, each containing a tag (C9) at its C terminus, and an anti-tag antibody. Immunoprecipitates were analysed by SDS–PAGE. c, Labelled Vero E6 cell lysates were immunoprecipitated with S1–Ig or soluble IFNAR2–Ig. Immunoprecipitates were treated or not, as indicated, with PNGase F, and analysed by SDS–PAGE.
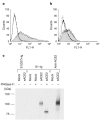
Figure 2. A high-affinity association between ACE2 and the S1 domain.
a, 293T cells transfected with plasmid encoding ACE2 (shaded area and dotted line), or with vector alone (thick solid line), were analysed by flow cytometry with S1–Ig (shaded area and thick solid line) or S1(327)–Ig (dotted line). b, Vero E6 cells were incubated with S1–Ig (shaded area, thin solid line and thick solid line), or with medium alone (dotted line), in the presence of soluble ACE1 (thin solid line) or ACE2 (thick solid line), or without either protein (shaded area and dotted line). c, Supernatants of radiolabelled 293T cells transfected with plasmid encoding soluble ACE2 or with vector alone (mock) were immunoprecipitated with S1(327)–Ig, S1–Ig, or an anti-ACE2 antibody. Immunoprecipitates were treated or not, as indicated, with PNGase F, and analysed by SDS–PAGE.
Figure 3. Syncytia formation between S-protein- and ACE2-expressing cells.
a, 293T cells transfected with plasmids encoding the HIV-1 envelope glycoprotein gp160 (top row) or SARS-CoV S protein (bottom row) were mixed at a 1:1 ratio with 293T cells transfected with plasmids encoding HIV-1 receptors CD4 and CCR5 (left column) or ACE2 (right column). b, 293T cells transfected with ACE2 plasmid were mixed at a 1:1 ratio with 293T cells transfected with plasmid encoding S protein, in the presence of 10 µg ml-1 goat polyclonal control antibody (left) or goat polyclonal anti-ACE2 antibody (right). Ab, antibody.
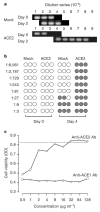
Figure 4. Efficient replication of SARS-CoV in the presence of ACE2.
a, Mock- or ACE2-transfected 293T cells were infected with SARS-CoV for 1 h, and cell supernatants sampled 0 and 48 h after washing. Viral RNA was measured by RT–PCR at varying dilutions of supernatant. The endpoint of dilution is shown for each group. b, Supernatants (collected at days 0 and 4) of infected mock- and ACE2-transfected 293T cells were incubated at the indicated dilutions in triplicate with Vero E6 cells plated on 96-well microtitre plates. Cytopathic effect, indicated by a shaded circle, was monitored for each well 3 days after Vero E6 cell infection. c, Duplicate samples of Vero E6 cells were incubated with affinity-purified goat anti-ACE1 or anti-ACE2 antibody, at the indicated concentrations, before infection with SARS-CoV. Cells were washed and cell viability, shown as the average optical density (OD) at 490 nm wavelength, was assayed using CellTiter 96. The dotted line indicates viability of uninfected cells. No toxicity was observed in uninfected cells treated with the maximum concentration of either antibody.
Similar articles
- Retroviruses pseudotyped with the severe acute respiratory syndrome coronavirus spike protein efficiently infect cells expressing angiotensin-converting enzyme 2.
Moore MJ, Dorfman T, Li W, Wong SK, Li Y, Kuhn JH, Coderre J, Vasilieva N, Han Z, Greenough TC, Farzan M, Choe H. Moore MJ, et al. J Virol. 2004 Oct;78(19):10628-35. doi: 10.1128/JVI.78.19.10628-10635.2004. J Virol. 2004. PMID: 15367630 Free PMC article. - Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63.
Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, Simmons G, Hofmann H, Kuri T, Weber F, Eichler J, Drosten C, Pöhlmann S. Glowacka I, et al. J Virol. 2010 Jan;84(2):1198-205. doi: 10.1128/JVI.01248-09. Epub 2009 Oct 28. J Virol. 2010. PMID: 19864379 Free PMC article. - Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2.
Glende J, Schwegmann-Wessels C, Al-Falah M, Pfefferle S, Qu X, Deng H, Drosten C, Naim HY, Herrler G. Glende J, et al. Virology. 2008 Nov 25;381(2):215-21. doi: 10.1016/j.virol.2008.08.026. Epub 2008 Sep 23. Virology. 2008. PMID: 18814896 Free PMC article. - Insights from the association of SARS-CoV S-protein with its receptor, ACE2.
Li W, Choe H, Farzan M. Li W, et al. Adv Exp Med Biol. 2006;581:209-18. doi: 10.1007/978-0-387-33012-9_36. Adv Exp Med Biol. 2006. PMID: 17037532 Free PMC article. Review. No abstract available. - Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus.
Kuhn JH, Li W, Choe H, Farzan M. Kuhn JH, et al. Cell Mol Life Sci. 2004 Nov;61(21):2738-43. doi: 10.1007/s00018-004-4242-5. Cell Mol Life Sci. 2004. PMID: 15549175 Free PMC article. Review.
Cited by
- Analyzing prognosis and comparing predictive scoring systems for mortality of COVID-19 patients with liver cirrhosis: a multicenter retrospective study.
Chen SY, Ng CJ, Huang YB, Lo HY. Chen SY, et al. BMC Infect Dis. 2024 Nov 18;24(1):1315. doi: 10.1186/s12879-024-10223-4. BMC Infect Dis. 2024. PMID: 39558236 Free PMC article. - ACE2-independent sarbecovirus cell entry can be supported by TMPRSS2-related enzymes and can reduce sensitivity to antibody-mediated neutralization.
Zhang L, Cheng HH, Krüger N, Hörnich B, Graichen L, Hahn AS, Schulz SR, Jäck HM, Stankov MV, Behrens GMN, Müller MA, Drosten C, Mörer O, Winkler MS, Qian Z, Pöhlmann S, Hoffmann M. Zhang L, et al. PLoS Pathog. 2024 Nov 13;20(11):e1012653. doi: 10.1371/journal.ppat.1012653. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39536058 Free PMC article. - Using Zebrafish to Study Multiciliated Cell Development and Disease States.
Nguyen TK, Baker S, Rodriguez JM, Arceri L, Wingert RA. Nguyen TK, et al. Cells. 2024 Oct 23;13(21):1749. doi: 10.3390/cells13211749. Cells. 2024. PMID: 39513856 Free PMC article. Review. - A novel neutralizing monoclonal antibody recognizes a linear antigenic epitope of the spike protein of swine acute diarrhoea syndrome coronavirus.
Zhang L, Liu HZ, Lian Y, Zhu Y, Wu M, Liu J, Cong F. Zhang L, et al. Virol J. 2024 Nov 6;21(1):279. doi: 10.1186/s12985-024-02562-0. Virol J. 2024. PMID: 39501289 Free PMC article. - Delineating the functional activity of antibodies with cross-reactivity to SARS-CoV-2, SARS-CoV-1 and related sarbecoviruses.
Ruiz F, Foreman WB, Lilly M, Baharani VA, Depierreux DM, Chohan V, Taylor AL, Guenthoer J, Ralph D, Matsen Iv FA, Chu HY, Bieniasz PD, Côté M, Starr TN, Overbaugh J. Ruiz F, et al. PLoS Pathog. 2024 Oct 28;20(10):e1012650. doi: 10.1371/journal.ppat.1012650. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39466880 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous