The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli - PubMed (original) (raw)
The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli
J Amiel Rosenkranz et al. J Neurosci. 2003.
Abstract
The amygdala plays a role in learning and memory processes that involve an emotional component. However, neural structures that regulate these amygdala-dependent processes are unknown. Previous studies indicate that regulation of affect may be imposed by the prefrontal cortex (PFC) and its efferents to the amygdala. The presentation of conditioned affective stimuli enhances activity of neurons in the lateral nucleus of the amygdala (LAT), which is thought to drive conditioned affective responses. Moreover, plasticity of LAT neuronal responses to stimuli during the course of conditioning is believed to underlie affective learning. This study examines the role of the PFC in the regulation of affective behaviors by evaluating how the PFC affects LAT neuronal plasticity and activity that is evoked by previously conditioned stimuli. In vivo intracellular recordings were performed from the LAT of anesthetized rats during pavlovian conditioning and during the presentation of stimuli that were conditioned in the awake rat before recording. Train stimulation of the PFC suppressed LAT neuronal activity that was evoked by both previously conditioned and neutral stimuli. In addition, PFC stimulation blocked LAT neuronal plasticity associated with an affective conditioning procedure. These results indicate that the PFC has the potential to regulate affective processes by inhibition of the LAT. Patients with disruptions of the PFC-LAT interaction often display an inability to regulate affective responses. This may be attributable to the loss of PFC-imposed inhibition of the emotional response to a stimulus but may also include the formation or diminished extinction of inappropriate associations.
Figures
Figure 1.
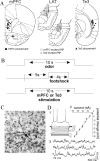
Neuron locations and electrode placements. A, Stimulation electrodes were placed in the mPFC (infralimbic and prelimbic cortices; filled circles) and Te3 (filled triangles). Stimulation of the mPFC and Te3 alters the activity of neurons in the LAT (open circles and open triangles, respectively). B, The conditioning procedure in anesthetized rats included the pairing of a 10 sec odor presentation with a 4 sec footshock. When noted, the mPFC or Te3 was stimulated during the odor-footshock pairings. C, Morphology of biocytin-filled LAT neurons is consistent with projection neurons (20× magnification). D, The biocytin-filled neuron (in C) displays electrophysiological characteristics consistent with LAT projection neurons, including adapting firing pattern (1), input resistance (2; 47 MΩ), and membrane potential (3; -74 mV) with spontaneous PSPs.
Figure 2.
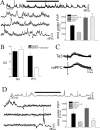
Stimulation of the mPFC suppresses spike firing. Te3 and mPFC stimulation exert opposite actions on membrane potential and action potential firing when the membrane is depolarized. A, Te3 train stimulation evokes membrane depolarization and spike firing (part of the voltage response, indicated by the solid horizontal bar, is expanded in bottom traces). B, Conversely, mPFC stimulation results in membrane hyperpolarization and cessation of spike firing.
Figure 3.
Stimulation of the mPFC suppresses sPSPs. A, Train stimulation of Te3 afferents evokes PSPs that summate with sPSPs. Stimulation is indicated by the horizontal bar in the top trace, which is expanded in the bottom traces. Note the occurrence of sPSPs during Te3 stimulation (arrows demark the start and end of stimulation). B, The lack of suppression of sPSPs by Te3 inputs can be demonstrated by the unchanged SD of the mean membrane potential and the linear summation of the area under the PSP (A, inset). C, PSPs of similar amplitudes evoked by mPFC and Te3 stimulation display similar decay times. D, When the mPFC is stimulated in trains, no summation is observed, and sPSPs in LAT neurons are suppressed. The expanded bottom traces demonstrate the abrupt cessation of sPSPs at the onset of mPFC stimulation (first arrow). This can be quantified as a reduction in the SD of the mean membrane potential (B) and a sublinear summation (D, inset). Stimulus artifacts have been removed from most traces for clarity in this and subsequent figures. *p < 0.05; t test (significant difference between the mPFC and the Te3); +p < 0.05; paired t test (significant difference between stimulation and baseline).
Figure 4.
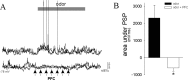
Stimulation of the mPFC suppresses responses to novel odors. Presentation of odors results in PSPs in many LAT neurons (A, top; odor was presented at the gray bar in all traces). Train stimulation of the mPFC (15 Hz, 0.2 mA, indicated by arrows) during the odor presentation suppresses the PSPs evoked by this odor (bottom traces; each set of traces represents two overlays of neuronal activity immediately before and during odor presentation). B, The mPFC stimulation significantly suppresses odor-evoked PSPs (*p < 0.05; paired t test), as quantified by the area under the PSP during baseline odor presentation and during odor presentation with mPFC stimulation.
Figure 5.
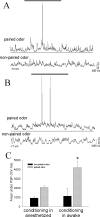
Stimulation of the mPFC suppresses responses to paired odors. Pairing of odors with footshock in awake rats results in an enhanced response of LAT neurons to the paired odors (A, top traces) recorded in anesthetized rats compared with nonpaired odors (B, top traces). Stimulation of the mPFC (15 Hz, 0.1 mA) during the odor presentation suppresses the response of this LAT neuron to both paired (A, bottom) and nonpaired (B, bottom) odors. Traces are overlays of the voltage response of a single LAT neuron to consecutive odor presentations (solid horizontal bar). C, As a group, paired odors evoke a greater response than nonpaired odors, and mPFC stimulation suppresses both odors to a similar extent compared with baseline odor-evoked responses, quantified as the area under the PSP. *p < 0.05; t test (paired odor compared with nonpaired odor); +p < 0.05; t test (baseline response to odor compared with response to odor in the presence of mPFC stimulation). D, The training procedure also resulted in differential behavioral responses to footshock-paired and nonpaired odors. These rats display a suppression of exploration in the presence of the footshock-paired odor (CS+) compared with the nonpaired odor (CS-; +p < 0.02; Wilcoxon signed rank test), as well as a suppression of grooming (*p < 0.001; binomial test); some rats displayed freezing (*p < 0.05; binomial test).
Figure 6.
BLA neurons exhibit significantly greater responses to odors that were conditioned previously in the awake rat compared with the response to odors conditioned concurrently with recordings under anesthesia. A, After an odor has been paired with footshock in an anesthetized rat, a noticeable response to the paired odor develops (top trace; gray bar above traces denotes duration of odor presentation in all traces), whereas the nonpaired odor evokes no obvious response from this LAT neuron (bottom trace). B, When the odor has been paired with footshock in awake rats, the paired odor evokes a robust response from LAT neurons (top trace; the thin line beneath traces is used to facilitate comparison between the response to odors that were conditioned in awake and anesthetized rats) recorded from anesthetized rats 24 hr after the conditioning procedure. The nonpaired odor does not evoke an obvious response (bottom trace). C, The activity of LAT neurons, measured as the area under the odor-evoked PSPs, is significantly greater in response to odors conditioned in awake rats compared with odors conditioned in anesthetized rats (*p < 0.05; t test). The response to nonpaired odors is not significantly different.
Figure 7.
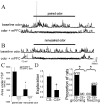
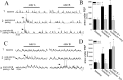
Stimulation of the mPFC suppresses the plasticity of LAT neurons. A, In anesthetized rats, an initially minimal response of a LAT neuron to an odor (1; presentation indicated by the gray bar) is enhanced after pairing of that odor with footshock (2, odor A, arrow), whereas the nonpaired odor does not display enhancement (2, odor B). Additional plasticity is blocked by stimulation of the mPFC during the odor-footshock pairings (3, odor B), whereas the previous enhancement of odor A is not reversed (3, odor A; compare arrows in the odor A and odor B traces in A3). B, The pairing procedure enhances a footshock-paired odor but not the nonpaired odor, and mPFC stimulation suppresses this plasticity (*p < 0.05 compared with baseline; +p < 0.05 compared with other odor), as quantified by the area under the PSP. C, Stimulation of the Te3, however, does not suppress this form of plasticity. Similar to the example above, this neuron does not display a noticeable response to odors (presented at bar, top traces). However, pairing of odor A with footshock results in an enhanced response to odor A (middle traces, arrow) but not odor B. Unlike stimulation of the mPFC, stimulation of the Te3 during the conditioning phase does not suppress the plasticity, as reflected by the enhanced response to odor B (the odor paired with footshock and Te3 stimulation). D, When quantified by the area under the PSP, as a group, it can be seen that the response of LAT neurons to paired odors is enhanced after the odor is paired with footshock. Furthermore, the enhancement of footshock-paired odors is not disrupted by Te3 train stimulation.
Similar articles
- Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning.
Rosenkranz JA, Grace AA. Rosenkranz JA, et al. Nature. 2002 May 16;417(6886):282-7. doi: 10.1038/417282a. Nature. 2002. PMID: 12015602 - Regulation of conditioned responses of basolateral amygdala neurons.
Grace AA, Rosenkranz JA. Grace AA, et al. Physiol Behav. 2002 Dec;77(4-5):489-93. doi: 10.1016/s0031-9384(02)00909-5. Physiol Behav. 2002. PMID: 12526988 Review. - Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo.
Rosenkranz JA, Grace AA. Rosenkranz JA, et al. J Neurosci. 2002 Jan 1;22(1):324-37. doi: 10.1523/JNEUROSCI.22-01-00324.2002. J Neurosci. 2002. PMID: 11756516 Free PMC article. - Neuronal responses of the rat amygdala during extinction and reassociation learning in elementary and configural associative tasks.
Toyomitsu Y, Nishijo H, Uwano T, Kuratsu J, Ono T. Toyomitsu Y, et al. Eur J Neurosci. 2002 Feb;15(4):753-68. doi: 10.1046/j.1460-9568.2002.01889.x. Eur J Neurosci. 2002. PMID: 11886454 - [Neural mechanisms of intelligence, emotion, and intention].
Ono T, Nishijo H. Ono T, et al. Brain Nerve. 2008 Sep;60(9):995-1007. Brain Nerve. 2008. PMID: 18807934 Review. Japanese.
Cited by
- Targeting neuronal nitric oxide synthase and the nitrergic system in post-traumatic stress disorder.
Sadeghi MA, Hemmati S, Nassireslami E, Yousefi Zoshk M, Hosseini Y, Abbasian K, Chamanara M. Sadeghi MA, et al. Psychopharmacology (Berl). 2022 Oct;239(10):3057-3082. doi: 10.1007/s00213-022-06212-7. Epub 2022 Aug 27. Psychopharmacology (Berl). 2022. PMID: 36029333 Review. - The influence of acute stress on the regulation of conditioned fear.
Raio CM, Phelps EA. Raio CM, et al. Neurobiol Stress. 2014 Nov 15;1:134-46. doi: 10.1016/j.ynstr.2014.11.004. eCollection 2015 Jan. Neurobiol Stress. 2014. PMID: 25530986 Free PMC article. Review. - Neural changes in extinction recall following prolonged exposure treatment for PTSD: A longitudinal fMRI study.
Helpman L, Marin MF, Papini S, Zhu X, Sullivan GM, Schneier F, Neria M, Shvil E, Malaga Aragon MJ, Markowitz JC, Lindquist MA, Wager T, Milad M, Neria Y. Helpman L, et al. Neuroimage Clin. 2016 Oct 10;12:715-723. doi: 10.1016/j.nicl.2016.10.007. eCollection 2016. Neuroimage Clin. 2016. PMID: 27761402 Free PMC article. - Affective network and default mode network in depressive adolescents with disruptive behaviors.
Kim SM, Park SY, Kim YI, Son YD, Chung US, Min KJ, Han DH. Kim SM, et al. Neuropsychiatr Dis Treat. 2015 Dec 31;12:49-56. doi: 10.2147/NDT.S95541. eCollection 2016. Neuropsychiatr Dis Treat. 2015. PMID: 26770059 Free PMC article. - The Relationship Between Facial Expression and Cognitive Function in Patients With Depression.
Ruihua M, Hua G, Meng Z, Nan C, Panqi L, Sijia L, Jing S, Yunlong T, Shuping T, Fude Y, Li T, Zhiren W. Ruihua M, et al. Front Psychol. 2021 Jun 21;12:648346. doi: 10.3389/fpsyg.2021.648346. eCollection 2021. Front Psychol. 2021. PMID: 34234708 Free PMC article.
References
- al Maskati HA, Zbrozyna AW ( 1989) Stimulation in prefrontal cortex area inhibits cardiovascular and motor components of the defense reaction in rats. J Auton Nerv Syst 28: 117-125. - PubMed
- Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RS, Dolan RJ ( 2002) Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel-based morphometry. Am J Psychiatry 159: 1497-1505. - PubMed
- Anderson SW, Damasio H, Tranel D, Damasio AR ( 2000) Long-term sequelae of prefrontal cortex damage acquired in early childhood. Dev Neuropsychol 18: 281-296. - PubMed
Publication types
MeSH terms
Grants and funding
- DA15408/DA/NIDA NIH HHS/United States
- F32 MH011305/MH/NIMH NIH HHS/United States
- R56 DA015408/DA/NIDA NIH HHS/United States
- R37 MH057440/MH/NIMH NIH HHS/United States
- R01 MH057440/MH/NIMH NIH HHS/United States
- R01 DA015408/DA/NIDA NIH HHS/United States
- F31 MH012533/MH/NIMH NIH HHS/United States
- MH12533/MH/NIMH NIH HHS/United States
- MH57440/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous