Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor - PubMed (original) (raw)
Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor
Zhenyu Yue et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The biochemical properties of beclin 1 suggest a role in two fundamentally important cell biological pathways: autophagy and apoptosis. We show here that beclin 1-/- mutant mice die early in embryogenesis and beclin 1+/- mutant mice suffer from a high incidence of spontaneous tumors. These tumors continue to express wild-type beclin 1 mRNA and protein, establishing that beclin 1 is a haploinsufficient tumor suppressor gene. Beclin 1-/- embryonic stem cells have a severely altered autophagic response, whereas their apoptotic response to serum withdrawal or UV light is normal. These results demonstrate that beclin 1 is a critical component of mammalian autophagy and establish a role for autophagy in tumor suppression. They both provide a biological explanation for recent evidence implicating beclin 1 in human cancer and suggest that mutations in other genes operating in this pathway may contribute to tumor formation through deregulation of autophagy.
Figures
Fig. 1.
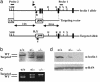
Targeted disruption of beclin 1 in embryonic cells. (a) Genomic structure of beclin 1 (showing exons 1–4 only; solid box), targeting vector, and beclin 1 allele after targeted deletion. The start codon is indicated by an asterisk. Arrow ”a” represents a common primer for both the wt and targeted beclin 1 alleles, arrow ”b” represents a specific primer for the wt allele, and arrow ”c” represents a specific primer for the targeted allele. X, _Xba_I; H, _Hin_dIII; R, _Eco_RI; B, _Bam_HI; neor, neomycin resistance cassette; TK, thymidine kinase marker. (b) Southern blot analysis of beclin 1 mutant ES clones after digestion with _Xba_I. The probe used to distinguish wt and targeted allele is indicated in a. wt allele is detected at a size of 11 kb, and mutant allele is detected at 7 kb. (c) Competitive PCR assay of three different genotypes of ES cells (+/+, +/-, and -/-) with primers a–c described in a. wt allele (250 bp) can be separated from mutated allele (500 bp) on a 1.5% agarose gel. (d) Western blot study of beclin 1 expression in ES cells from three different genotypes with anti-beclin 1 antibody. Anti-RAN antibody was used for protein loading control in each lane.
Fig. 2.
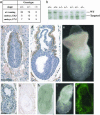
Disruption of beclin 1 causes early embryonic lethality. (a) Genotype distribution of offspring and embryos from beclin 1+/- intercrosses examined by Southern blotting or PCR. (b) Southern blot analysis of mouse genotype at age of weaning from a litter born by heterozygous parents. (c and d) Immunohistochemical analysis of beclin 1 expression in wt or heterozygous embryo at E6.5 and E7.5, respectively. Sections were counterstained with hematoxylin. (e) Image of wt whole embryo at E7.5. c_–_e are at the same magnification with a scale bar of 100 μm. (f and g) Sections from the same null mutant embryo (E7.5) were immunostained with anti-beclin 1 antibody and counterstained with (f) or without (g) hematoxylin. (h) Whole embryo of null mutant (E7.5) viewed under a Zeiss Axiovert confocal microscope with differential interference contrast microscopy. (i) The same embryo as in h viewed under serological microscope. (j) Whole embryo of null mutant stained with acridine orange and viewed with fluorescence under a Zeiss confocal microscope. f_–_j are at the same magnification with a scale bar of 20 μm.
Fig. 3.
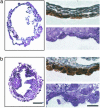
Disruption of beclin 1 causes abnormal formation and cell growth in VE of EB. Analysis of EBs (day 14) derived from ES cells of wt (a) and beclin 1-/- (b) is shown. (a and b, Left) H&E staining of paraffin sections. Note the expanded cystic form for wt and the cystic cavitated form for beclin 1-/-. (Scale bar, 100 μm.) (a and b, Right) Sections immunostained with anti-amnionless antibody (Upper) and toluidene blue-stained, 1-μm sections (Lower). (Scale bar, 10 μm.)
Fig. 4.
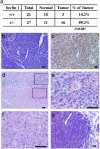
Increase of cancer rate in beclin 1+/- mice. (a) Summary of cancer incidents and cancer rate in wt and beclin 1+/- mice (age and gender matched). (b and c) B cell lymphoma. (b) H&E staining of tumor tissue. (c) Immunohistochemical staining of the same tumor in b with antibody against B220 (B cell marker). (d_–_f) Heptocellular carcinoma. (d) H&E staining of liver tissue containing heptocellular carcinoma. Note the upper portion showing normal tissue and the lower portion showing tumor. (e and f) High magnitude of normal and tumor tissue, respectively, in d.(g) Lung adenocarcinoma; H&E staining showing tumor (left portion) and normal (right portion) tissue of the lung. (Scale bar, 50 μm.)
Fig. 5.
Expression of beclin 1 protein in tumors from beclin 1+/- mice. (a) Western blot analysis of cell extracts from tumors with anti-beclin 1 antibody or anti-Ran antibody (control). Lane 1, cell lysate from wt spleen; lanes 2–4, B cell lymphomas; lane 5, lung adenocarcinoma; lane 6, heptocellular carcinoma. (Lower) Ran protein level in each lane as loading control. (b) Section from B cell lymphoma stained with (Left) or without (Right) anti-beclin 1 antibody. (c) Section from lung adenocarcinoma stained with (Left) or without (Right) anti-beclin 1 antibody. (Scale bar, 20 μm.)
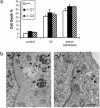
Fig. 6.
Disruption of beclin 1 causes autophagy deficiency. (a) Apoptotic cell death of wt or beclin 1-/- ES cells induced by UV irradiation or serum withdrawal. Mean values ± SEM are depicted (n = 3). C1 and C2 represent two independent beclin 1-/- ES clones. (b) Ultrastructural examination of ES cells after nutrient deprivation from beclin 1 wt (Left) and null mutant (Right) by electronic microscopy. Arrows indicate autophagy vacuoles. N, nucleus. (Scale bar, 1 μm.)
Comment in
- The importance of 'self-eating'.
Wrighton KH. Wrighton KH. Nat Rev Mol Cell Biol. 2010 Oct;11(10):681. doi: 10.1038/nrm2978. Nat Rev Mol Cell Biol. 2010. PMID: 20861878 No abstract available.
Similar articles
- Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene.
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Qu X, et al. J Clin Invest. 2003 Dec;112(12):1809-20. doi: 10.1172/JCI20039. Epub 2003 Nov 24. J Clin Invest. 2003. PMID: 14638851 Free PMC article. - Beclin 1 contains a leucine-rich nuclear export signal that is required for its autophagy and tumor suppressor function.
Liang XH, Yu J, Brown K, Levine B. Liang XH, et al. Cancer Res. 2001 Apr 15;61(8):3443-9. Cancer Res. 2001. PMID: 11309306 - Beclin-1 expression is retained in high-grade serous ovarian cancer yet is not essential for autophagy induction in vitro.
Correa RJ, Valdes YR, Shepherd TG, DiMattia GE. Correa RJ, et al. J Ovarian Res. 2015 Aug 4;8:52. doi: 10.1186/s13048-015-0182-y. J Ovarian Res. 2015. PMID: 26239434 Free PMC article. - Bcl-2 inhibition of autophagy: a new route to cancer?
Pattingre S, Levine B. Pattingre S, et al. Cancer Res. 2006 Mar 15;66(6):2885-8. doi: 10.1158/0008-5472.CAN-05-4412. Cancer Res. 2006. PMID: 16540632 Review. - [Analysis of cell cycle inhibition by novel tumor suppressor gene Beclin 1 in human colon cancer cells].
Koneri K, Goi T, Yamaguchi A. Koneri K, et al. Nihon Rinsho. 2003 Sep;61 Suppl 7:247-51. Nihon Rinsho. 2003. PMID: 14574891 Review. Japanese. No abstract available.
Cited by
- Mitochondrial biogenesis in epithelial cancer cells promotes breast cancer tumor growth and confers autophagy resistance.
Salem AF, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Salem AF, et al. Cell Cycle. 2012 Nov 15;11(22):4174-80. doi: 10.4161/cc.22376. Epub 2012 Oct 15. Cell Cycle. 2012. PMID: 23070475 Free PMC article. - Bioactive peptide isolated from sesame seeds inhibits cell proliferation and induces apoptosis and autophagy in leukemic cells.
Deesrisak K, Yingchutrakul Y, Krobthong S, Roytrakul S, Chatupheeraphat C, Subkorn P, Anurathapan U, Tanyong D. Deesrisak K, et al. EXCLI J. 2021 Mar 23;20:709-721. doi: 10.17179/excli2021-3406. eCollection 2021. EXCLI J. 2021. PMID: 33907539 Free PMC article. - Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy.
Park JM, Huang S, Wu TT, Foster NR, Sinicrope FA. Park JM, et al. Cancer Biol Ther. 2013 Feb;14(2):100-7. doi: 10.4161/cbt.22954. Epub 2012 Nov 28. Cancer Biol Ther. 2013. PMID: 23192274 Free PMC article. - Increased ATG5-ATG12 in hepatitis B virus-associated hepatocellular carcinoma and their role in apoptosis.
Kunanopparat A, Kimkong I, Palaga T, Tangkijvanich P, Sirichindakul B, Hirankarn N. Kunanopparat A, et al. World J Gastroenterol. 2016 Oct 7;22(37):8361-8374. doi: 10.3748/wjg.v22.i37.8361. World J Gastroenterol. 2016. PMID: 27729742 Free PMC article. - The Roles of Autophagy-related miRNAs in Gynecologic Tumors: A Review of Current Knowledge for Possible Targeted Therapy.
Mobinikhaledi M, Faridzadeh A, Farkhondeh T, Pourhanifeh MH, Samarghandian S. Mobinikhaledi M, et al. Curr Mol Med. 2024;24(10):1269-1281. doi: 10.2174/0115665240263059231002093454. Curr Mol Med. 2024. PMID: 39300715 Review.
References
- Ohsumi, Y. (2001) Nat. Rev. Mol. Cell Biol. 2, 211-216. - PubMed
- Khalfan, W. A. & Klionsky, D. J. (2002) Curr. Opin. Cell Biol. 14, 468-475. - PubMed
- Bursch, W. (2001) Cell Death Differ. 8, 569-581. - PubMed
- Xue, L., Fletcher, G. C. & Tolkovsky, A. M. (1999) Mol. Cell. Neurosci. 14, 180-198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials