Mast cells are required for experimental oral allergen-induced diarrhea - PubMed (original) (raw)
Mast cells are required for experimental oral allergen-induced diarrhea
Eric B Brandt et al. J Clin Invest. 2003 Dec.
Abstract
Gastrointestinal allergic disorders represent a diverse spectrum of inflammatory diseases that are occurring with increasing incidence and severity. An essential question concerning these disorders is to determine the specific cells and mediators responsible for specific clinical manifestations. With this in mind, we developed a murine model of oral allergen-induced intestinal inflammation accompanied by strong Th2-associated humoral and cellular responses and focused on the immunopathogenesis of allergic diarrhea. Exposure of OVA/alum-sensitized mice to repeated doses of intragastric OVA induced genetically restricted, dose-dependent, acute diarrhea associated with increased intestinal permeability, eosinophilia, and mastocytosis. Mice developed limited systemic manifestations of anaphylaxis, even though they developed marked intestinal mucosal mast cell degranulation. Notably, experiments involving mast cell depletion (with anti-c-kit mAb), anti-IgE treatment, and Fc epsilon RI-deficient mice indicated a critical effector role for mast cells in mediating allergic diarrhea. Furthermore, allergic diarrhea was dependent upon synergistic signaling induced by serotonin and platelet-activating factor (PAF), but not histamine. These results demonstrate that oral allergen-induced diarrhea associated with experimental Th2 intestinal inflammation is largely mast cell, IgE, serotonin, and PAF dependent.
Figures
Figure 1
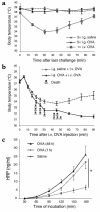
A murine model of allergen-induced diarrhea. (a) The time line illustrates that mice were sensitized with two OVA/alum intraperitoneal (i.p.) injections (vertical hatch mark) and subsequently treated with intragastric (i.g.) saline or OVA challenges every 2–3 days (vertical lines). (b) Diarrhea occurrence was assessed 15–45 minutes after intragastric challenges with either saline or increasing doses of OVA (2–50 mg in 250 μl of saline). (c) Diarrhea occurrence in BALB/c mice (n = 16) was compared with C57Bl/6 mice (n = 8) following ten intragastric OVA challenges (50 mg). (d) Representative photograph of the cecum and colon of BALB/c mice 60 minutes after ten intragastric saline or OVA challenges (upper and lower two samples, respectively). (e) Nonsensitized mice (n = 8) or OVA/alum–sensitized mice (n = 15) were treated with repeated doses of intragastric OVA (50 mg). Following five doses of OVA, sensitized mice were challenged with BSA (50 mg) as a control before resuming OVA challenges.
Figure 2
Clinical features associated with allergic diarrhea. (a) Anaphylaxis was assessed by measuring hypothermia following either five intragastric saline or OVA challenges. As a control, systemic anaphylaxis was induced in OVA/alum–sensitized mice by an i.v. OVA (100 μg) injection. Body temperatures are expressed in degrees Celsius as mean ± SEM (n = 8 mice/group). (b) Systemic anaphylaxis was also induced in OVA/alum–sensitized and saline- (n = 7) or OVA- (n = 13) intragastric challenged mice by an intravenous injection of OVA (100 μg). Death occurred only in the intragastric OVA group (11 out of 13 mice). (c) Jejunum segments were isolated 1 hour after five to six saline or OVA challenges, and intestinal permeability to HRP was measured in an Ussing chamber over a 180-minute period (mean ± SEM; n = 8 mice/group; *P = 0.0012).
Figure 3
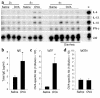
Intestinal and systemic Th2 responses. (a) Jejunum samples were collected 60 minutes after two, three, or four saline or OVA challenges and analyzed by RPA for Th1/Th2 cytokine expression. The positive control (+) (see Methods) expressed IL-4, IL-10, and IFN-γ. The housekeeping gene L32 was used as a control for RNA loading. Total IgE (b), OVA-specific IgG1 (c), and IgG2a (d) plasma titers were measured following OVA or saline challenges (mean ± SEM; n = 8–11 mice/group; *P = 0.020; #P = 0.003.)
Figure 4
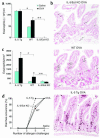
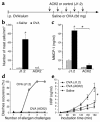
Role of eosinophils in allergic diarrhea. (a) Blood eosinophil levels in IL-5 transgenic (Tg) mice, IL-5/eotaxin-1 double-deficient mice (IL-5/Eot KO), and WT BALB/c mice were assessed 72 hours after the third saline or OVA challenge (mean ± SEM; n = 4–10 mice; *P < 0.0001; #P < 0.004; **P = 0.0035) (b) Representative jejunum section from OVA-challenged WT, IL-5 transgenic, and IL-5/eotaxin-1 double-deficient mice, immunostained with anti-MBP Ab as described in Methods (arrows indicate eosinophils; original magnification, ×125). (c) Jejunum eosinophil levels were assessed by morphometric analysis in IL-5 transgenic mice, IL-5/eotaxin-1 double-deficient mice, and WT BALB/c mice (mean ± SEM; n = 4 mice; *P < 0.05; #P = 0.005; **P = 0.004). (d) Diarrhea occurrence in IL-5 transgenic, IL-5/eotaxin-1 double-deficient, and WT control mice following saline or OVA challenges.
Figure 5
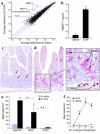
Mucosal mast cell responses in allergic diarrhea. (a) A scatter plot comparing the average difference in gene expression measured on the murine U74Av2 GeneChip following ten saline or OVA challenges was generated using GeneSpring software. The three lines indicate a twofold increase or twofold decrease compared with the mean. Representative, highly increased mast cell–related genes following diarrhea are indicated. (b) MMCP-1 plasma levels were measured by ELISA following saline or OVA challenges. Representative fields (original magnification, ×125) of saline-challenged (c) or OVA-challenged (d) jejunum sections illustrate chloroaces) indicated by arrows (original magnification, ×500). (e) Mucosal mast cell levels in the jejunum, ileum, and colon following three saline or OVA challenges were assessed by morphometric analysis of chloroacetate esterase–stained cells (n = 3–4 mice per group; #P < 0.0001; ##P = 0.013; *P = 0.032; **P = 0.003). (f) A kinetic analysis of mast cell recruitment to the jejunum of saline- or OVA-challenged mice (#P < 0.0005).
Figure 6
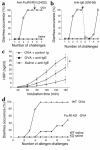
Absence of diarrhea in mast cell–depleted mice. (a) Following sensitization, mice were treated with a neutralizing ACK2 or a control Ab (J1.2) 24 hours before each intragastric challenge (arrows). Each vertical line represents saline or allergen challenge every 2–3 days. The efficacy of the treatment was assessed by chloroacetate esterase staining for jejunum mast cells (b) and measuring plasma MMCP-1 levels (c) 60 minutes after the fifth saline or OVA challenge in mice treated with either ACK2 or J1.2 (n = 4; #P < 0.005; *P = 0.0026). (d) Occurrence of diarrhea following five OVA challenges in control Ab (J1.2) or ACK2-treated mice. (e) Intestinal permeability to HRP was measured in an Ussing chamber over 180 minutes (mean ± SEM; n = 4 mice/group; *P = 0.01). n.d., not detected.
Figure 7
Allergic diarrhea is IgE mediated. Following two OVA/alum sensitizations, mice were treated with intragastric saline or OVA challenges as depicted in Figure 1a. Twenty-four hours before challenge six (arrow), mice were treated with either an Ab against (a) the low-affinity IgG receptors FcγRII/RIII (24G2) or (b) with an anti-IgE Ab (EM-95) (n = 6–8 mice; *P = 0.033). (c) Mice were treated with the EM-95 or GL117 control Ab (mean ± SEM; n = 4–6 mice), and intestinal permeability to HRP was measured in an Ussing chamber over 180 minutes. (d) Diarrhea occurrence in FcεRI KO mice and WT littermate controls following up to ten intragastric saline or OVA challenges (n = 6 and 5, respectively).
Similar articles
- Curcumin Ingestion Inhibits Mastocytosis and Suppresses Intestinal Anaphylaxis in a Murine Model of Food Allergy.
Kinney SR, Carlson L, Ser-Dolansky J, Thompson C, Shah S, Gambrah A, Xing W, Schneider SS, Mathias CB. Kinney SR, et al. PLoS One. 2015 Jul 6;10(7):e0132467. doi: 10.1371/journal.pone.0132467. eCollection 2015. PLoS One. 2015. PMID: 26147007 Free PMC article. - IL-10 Enhances IgE-Mediated Mast Cell Responses and Is Essential for the Development of Experimental Food Allergy in IL-10-Deficient Mice.
Polukort SH, Rovatti J, Carlson L, Thompson C, Ser-Dolansky J, Kinney SR, Schneider SS, Mathias CB. Polukort SH, et al. J Immunol. 2016 Jun 15;196(12):4865-76. doi: 10.4049/jimmunol.1600066. Epub 2016 May 6. J Immunol. 2016. PMID: 27183617 Free PMC article. - Allergen-specific antibody and cytokine responses, mast cell reactivity and intestinal permeability upon oral challenge of sensitized and tolerized mice.
Perrier C, Thierry AC, Mercenier A, Corthésy B. Perrier C, et al. Clin Exp Allergy. 2010 Jan;40(1):153-62. doi: 10.1111/j.1365-2222.2009.03329.x. Epub 2009 Aug 18. Clin Exp Allergy. 2010. PMID: 19689461 - Platelet activating factor in allergies.
Pałgan K, Bartuzi Z. Pałgan K, et al. Int J Immunopathol Pharmacol. 2015 Dec;28(4):584-9. doi: 10.1177/0394632015600598. Epub 2015 Oct 20. Int J Immunopathol Pharmacol. 2015. PMID: 26486136 Review. - Inhibition of the effector phase of IgE-mediated allergies by tolerogenic conjugates of allergens and monomethoxypolyethylene glycol.
Bitoh S, Wakefield R, Lang GM, Sehon AH. Bitoh S, et al. Int Arch Allergy Immunol. 1995 May-Jun;107(1-3):316-8. doi: 10.1159/000237012. Int Arch Allergy Immunol. 1995. PMID: 7613157 Review.
Cited by
- Physiopathological Roles of White Adiposity and Gut Functions in Neuroinflammation.
Spinedi E, Docena GH. Spinedi E, et al. Int J Mol Sci. 2024 Oct 31;25(21):11741. doi: 10.3390/ijms252111741. Int J Mol Sci. 2024. PMID: 39519291 Free PMC article. Review. - Immunotherapy with biodegradable nanoparticles encapsulating the oligosaccharide galactose-alpha-1,3-galactose enhance immune tolerance against alpha-gal sensitization in a murine model of alpha-gal syndrome.
Saunders MN, Rival CM, Mandal M, Cramton K, Rad LM, Janczak KW, Williams LA, Angadi AR, O'Konek JJ, Shea LD, Erickson LD. Saunders MN, et al. Front Allergy. 2024 Aug 9;5:1437523. doi: 10.3389/falgy.2024.1437523. eCollection 2024. Front Allergy. 2024. PMID: 39183976 Free PMC article. - New Insight into Intestinal Mast Cells Revealed by Single-Cell RNA Sequencing.
Putro E, Carnevale A, Marangio C, Fulci V, Paolini R, Molfetta R. Putro E, et al. Int J Mol Sci. 2024 May 21;25(11):5594. doi: 10.3390/ijms25115594. Int J Mol Sci. 2024. PMID: 38891782 Free PMC article. Review. - IL-10 Neutralization Attenuates Mast Cell Responses in a Murine Model of Experimental Food Allergy.
Krajewski D, Ranjitkar S, Tedeschi C, Perez NM, Jordan N, Mire M, Schneider SS, Mathias CB. Krajewski D, et al. Immunohorizons. 2024 Jun 1;8(6):431-441. doi: 10.4049/immunohorizons.2400002. Immunohorizons. 2024. PMID: 38888412 Free PMC article. - 5-HTP inhibits eosinophilia via intracellular endothelial 5-HTRs; SNPs in 5-HTRs associate with asthmatic lung function.
Walker MT, Bloodworth JC, Kountz TS, McCarty SL, Green JE, Ferrie RP, Campbell JA, Averill SH, Beckman KB, Grammer LC, Eng C, Avila PC, Farber HJ, Rodriguez-Cintron W, Rodriguez-Santana JR, Serebrisky D, Thyne SM, Seibold MA, Burchard EG, Kumar R, Cook-Mills JM. Walker MT, et al. Front Allergy. 2024 May 23;5:1385168. doi: 10.3389/falgy.2024.1385168. eCollection 2024. Front Allergy. 2024. PMID: 38845678 Free PMC article.
References
- Sampson HA. 9. Food allergy. J. Allergy Clin. Immunol. 2003; 111(Suppl. 2):S540–S547. - PubMed
- Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Gastrointestinal eosinophils in health and disease. Adv. Immunol. 2001; 78:291–328. - PubMed
- Kweon MN, Kiyono H. Eosinophilic gastroenteritis: a problem of the mucosal immune system? Curr. Allergy Asthma Rep. 2003; 3:79–85. - PubMed
- Crowe SE, Perdue MH. Gastrointestinal food hypersensitivity: basic mechanisms of pathophysiology. Gastroenterology. 1992; 103:1075–1095. - PubMed
- Lin RY, et al. Histamine and tryptase levels in patients with acute allergic reactions: an emergency department-based study. J. Allergy Clin. Immunol. 2000; 106:65–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01AI50006/AI/NIAID NIH HHS/United States
- R01 AI-45898-03/AI/NIAID NIH HHS/United States
- K08 AI-50006/AI/NIAID NIH HHS/United States
- R01 AI045898/AI/NIAID NIH HHS/United States
- R01 AI-42242-05/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical