Small GTPase Rin induces neurite outgrowth through Rac/Cdc42 and calmodulin in PC12 cells - PubMed (original) (raw)
Small GTPase Rin induces neurite outgrowth through Rac/Cdc42 and calmodulin in PC12 cells
Mitsunobu Hoshino et al. J Cell Biol. 2003.
Abstract
The novel Ras-like small GTPase Rin is expressed prominently in adult neurons, and binds calmodulin (CaM) through its COOH-terminal-binding motif. It might be involved in calcium/CaM-mediated neuronal signaling, but Rin-mediated signal transduction pathways have not yet been elucidated. Here, we show that expression of Rin induces neurite outgrowth without nerve growth factor or mitogen-activated protein kinase activation in rat pheochromocytoma PC12 cells. Rin-induced neurite outgrowth was markedly inhibited by coexpression with dominant negative Rac/Cdc42 protein or CaM inhibitor treatment. We also found that expression of Rin elevated the endogenous Rac/Cdc42 activity. Rin mutant proteins, in which the mutation disrupted association with CaM, failed to induce neurite outgrowth irrespective of Rac/Cdc42 activation. Disruption of endogenous Rin function inhibited the neurite outgrowth stimulated by forskolin and extracellular calcium entry through voltage-dependent calcium channel evoked by KCl. These findings suggest that Rin-mediated neurite outgrowth signaling requires not only endogenous Rac/Cdc42 activation but also Rin-CaM association, and that endogenous Rin is involved in calcium/CaM-mediated neuronal signaling pathways.
Figures
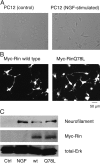
Figure 1.
Rin protein induces neurite outgrowth in PC12 cells. (A) Empty vector–transfected PC12 cells were left untreated or stimulated with 50 ng/ml NGF for 44 h. Cells were fixed with 3% PFA-PBS and were visualized under a phase contrasted bright field microscopy. (B) PC12 cells were transfected with an expression vector encoding Myc-tagged wild-type Rin or Myc-tagged constitutively active RinQ78L protein. After 48 h, transfected cells were fixed, permeabilized, and processed for immunofluorescence with an anti-Myc antibody. Transfected cells were visualized with a Cy3-labeled anti–mouse secondary antibody. (C) PC12 cells were left untreated or stimulated with 50 ng/ml NGF for 2 d. Other PC12 cells were transfected with an expression vector encoding Myc-tagged wild-type Rin or Myc-tagged RinQ78L protein and were maintained for 5 d. Cells were washed with an ice-cold PBS buffer and lysed with an ice-cold lysis buffer. Cell lysates were cleared by centrifugation and Western blotting was performed as described in the Materials and methods, using an antineurofilament antibody, an anti-MAPK 1/2 antibody, and an anti-Myc antibody. Data are representative of three independent experiments, which gave essentially identical results.
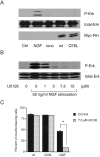
Figure 2.
MAPK is not involved in the Rin-signaling pathway in PC12 cells. (A) PC12 cells were serum starved for 24 h and left untreated or stimulated with 50 ng/ml NGF or 0.5 μg/ml ionomycin for 5 min. Other PC12 cells were transfected with an expression vector encoding Myc-tagged wild-type Rin or Myc-tagged RinQ78L protein and serum starved for 24 h. Cell lysates were subjected to Western blotting as described in the Materials and methods, using an antiphospho-MAPK antibody, an anti-MAPK 1/2 antibody and an anti-Myc antibody. Data are representative of three independent experiments, which gave essentially identical results. (B) Serum-starved PC12 cells were preincubated with vehicle or various amounts of MAPK kinase inhibitor U0126 for 48 h at 37°C, followed by 50 ng/ml NGF for 5 min. Cell lysates were subjected to Western blotting as described in the Materials and methods. Data are representative of three independent experiments, which gave essentially identical results. (C) Rin-transfected cells or 50 ng/ml NGF-stimulated cells were treated with vehicle or 7.5 μM U0126 for 48 h at 37°C. Cells were fixed and visualized with an anti-Myc antibody. Cells with neurites exceeding one cell body diameter in length were counted as a ratio of the total number of transfected cells. Columns and vertical bars denote the mean ± SEM, respectively (n = 3). Asterisk indicates P < 0.05 as compared with the control value.
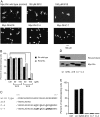
Figure 3.
Dominant negative Rac/Cdc42 inhibits the Rin-mediated neurite outgrowth in PC12 cells. (A and B) Cells were transfected with an HA-tagged wild-type Rin vector and a Myc-tagged dominant negative RacS17N/Cdc42S17N/RhoT19N vector either alone or in pairs. After 48 h, cells were fixed and immunostained with an anti-HA antibody and an anti-Myc antibody. Columns and vertical bars denote the mean ± SEM, respectively (n = 3). In the merge of A, HA-Rin staining is shown in green (using FITC-labeled anti–rat secondary antibody), whereas Myc-Rac staining is shown in red (using Cy3- labeled anti–mouse secondary antibody).
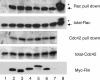
Figure 4.
Rin activates endogenous Rac/Cdc42 in PC12 cells. Cells were transfected with an empty vector or Myc-tagged expression vectors encoding several Rin proteins. After 48 h, cells were lysed and cleared, followed by Rac/Cdc42 pull-down assay, as described in the Materials and methods. Bound endogenous Rac proteins (arrow) and Cdc42 proteins were visualized by Western blotting using an anti-Rac antibody and anti-Cdc42 antibody, respectively. Lane 1, empty vector; lane 2, wild-type Myc-Rin, lane 3, Myc-RinQ78L; lane 4, Myc-RinΔ18; lane 5, Myc-RinC-4; lane 6, Myc-RinC-7; lane 7, empty vector with 50 ng/ml NGF for 3 min; lane 8, constitutively active Myc-Rac (positive control, asterisk). Data are representative of three independent experiments, which gave essentially identical results.
Figure 5.
CaM association is necessary for Rin-induced neurite outgrowth in PC12 cells. (A, B) PC12 cells were transfected with Myc-tagged expression vectors encoding several Rin proteins and incubated with vehicle, 50 or 100 μM W13 (CaM antagonist) or W12 (inactive analogue of W13). After 48 h, cells were fixed and immunostained with an anti-Myc antibody. Columns and vertical bars denote the mean ± SEM, respectively (n = 3). Asterisks indicate P < 0.05. (C) Comparison of the COOH-terminal amino acid sequences (amino acids 191–217) of wild-type Rin protein, RinΔ18, RinC-4, and RinC-7. The residues of the mutant proteins that are different from those of the wild-type Rin protein are italicized. (D) Cos-7 cells were transfected with Myc-tagged expression vectors encoding several Rin proteins. After 48 h, cells were lysed, cleared, and Rin proteins were pulled down with CaM-conjugated agarose beads at 4°C for 2 h as described in the Materials and methods. Bound Myc-Rin protein was visualized with an anti-Myc antibody. Data are representative of three independent experiments, which gave essentially identical results. (E) PC12 cells were transfected with Myc-tagged expression vectors encoding several Rin mutant proteins. After 48 h, cells were fixed and immunostained with an anti-Myc antibody. Columns and vertical bars denote the mean ± SEM, respectively (n = 3).
Figure 6.
Rin activates endogenous Rho in PC12 cells. (A) PC12 cells were transfected with an empty vector or Myc-tagged Rin vector. After 48 h, cells were stimulated with vehicle or 10 μM LPA for 1 min. Cells were lysed and cleared, followed by Rho activation assay, as described in the Materials and methods. Lane 1, empty vector; lane 2, wild-type Myc-Rin, lane 3, Myc-RinQ78L; lane 4, Myc-RinΔ18; lane 5, Myc-RinC-4; lane 6, Myc-RinC-7; lane 7, empty vector with 10 μM LPA for 1 min. Data are representative of three independent experiments, which gave essentially identical results. (B) PC12 cells were transfected with indicated amounts of siRNA of RhoA or control siRNA. After 48 h, cells were lysed, cleared and followed by Western blotting as described in the Materials and methods. (C) PC12 cells were transfected with a Myc-tagged wild-type Rin vector and a siRNA of RhoA either alone or in pairs. After 48 h, cells were fixed and observed as described in the Materials and methods.
Figure 7.
Endogenous Rin protein is involved in calcium-mediated neurite outgrowth in PC12 cells. (A) The expression of Rin and GAPDH (constitutively expressed gene) at the mRNA level was detected by performing RT-PCR from total RNA isolated from Cos-7, PC12, and primary dissociated neuronal cells from P3 mouse brain, as described in the Materials and methods. (B) PC12 cells were transfected with an HA-tagged wild-type Rin vector and a Myc-tagged vector encoding RinG29V-C-7 mutant protein either alone or in pairs. After 48 h, cells were fixed and immunostained with an anti-HA antibody and an anti-Myc antibody. In the merge, HA-Rin staining is shown in green (using FITC-labeled anti–rat secondary antibody), whereas Myc-Rin staining is shown in red (using Cy3-labeled anti–mouse secondary antibody). (C) PC12 cells were transfected with an empty vector or a Myc-tagged RinG29V-C-7 expression vector. After 4 h, transfected cells were stimulated with vehicle or 50 ng/ml NGF for 44 h. Cells were fixed and immunostained with an anti-Myc antibody. Columns and vertical bars denote the mean ± SEM, respectively (n = 3). (D) PC12 cells were transfected with an empty vector or RinG29V-C-7 expression vector. After 48 h, they were stimulated with vehicle or 50 ng/ml NGF for 5 min. Cells were lysed and cleared, followed by a Rac/Cdc42 pull-down assay (Rac PD and Cdc42 PD). Bound endogenous Rac proteins (arrow) and Cdc42 proteins were visualized by Western blotting. Constitutively active Myc-Rac (positive control) is indicated by asterisk. Data are representative of three independent experiments, which gave essentially identical results. (E) PC12 cells were transfected with an empty vector or a Myc-tagged RinG29V-C-7 expression vector. After 4 h, transfected cells were pretreated with vehicle or the L-type calcium channel blocker nitrendipine (final 10 μM)/diltiazem (final 50 μM) for 30 min and stimulated with 50 μM forskolin alone or 50 μM forskolin plus 50 mM KCl for 44 h. Cells were fixed and immunostained with an anti-Myc antibody. Columns and vertical bars denote the mean ± SEM, respectively (n = 3). Asterisks indicate P < 0.05. (F) PC12 cells were transfected with 1 μg of Myc-tagged wild-type Rin vector and indicated amounts of siRNA. After 48 h, cells were lysed, cleared and followed by Western blotting as described in the Materials and methods. (G) PC12 cells were transfected with 1 μg of pGFP-C1 vector and indicated amounts of siRNA. After 4 h, transfected cells were stimulated with 50 μM forskolin alone or 50 μM forskolin plus 50 mM KCl for 44 h. Cells were fixed and counted as described in the Materials and methods. Columns and vertical bars denote the mean ± SEM, respectively (n = 3). The asterisk indicates P < 0.05. (H) PC12 cells were transfected with 1 μg of Myc-tagged RacS17N/Cdc42S17N and 1 μg of pGFP-C1 vector plus 10 μg of Rin-specific siRNA either alone or in pairs. After 4 h, transfected cells were stimulated with 50 μM forskolin alone or 50 μM forskolin plus 50 mM KCl for 44 h. Cells were fixed and counted as described in the Materials and methods. Columns and vertical bars denote the mean ± SEM, respectively (n = 3). Asterisks indicate P < 0.05.
Similar articles
- RhoE stimulates neurite-like outgrowth in PC12 cells through inhibition of the RhoA/ROCK-I signalling.
Talens-Visconti R, Peris B, Guerri C, Guasch RM. Talens-Visconti R, et al. J Neurochem. 2010 Feb;112(4):1074-87. doi: 10.1111/j.1471-4159.2009.06526.x. Epub 2009 Dec 3. J Neurochem. 2010. PMID: 19968760 - Signaling mediated by the closely related mammalian Rho family GTPases TC10 and Cdc42 suggests distinct functional pathways.
Murphy GA, Jillian SA, Michaelson D, Philips MR, D'Eustachio P, Rush MG. Murphy GA, et al. Cell Growth Differ. 2001 Mar;12(3):157-67. Cell Growth Differ. 2001. PMID: 11306516 - The c-Fes tyrosine kinase cooperates with the breakpoint cluster region protein (Bcr) to induce neurite extension in a Rac- and Cdc42-dependent manner.
Laurent CE, Smithgall TE. Laurent CE, et al. Exp Cell Res. 2004 Sep 10;299(1):188-98. doi: 10.1016/j.yexcr.2004.05.010. Exp Cell Res. 2004. PMID: 15302586 - Role of the Go/i signaling network in the regulation of neurite outgrowth.
He JC, Neves SR, Jordan JD, Iyengar R. He JC, et al. Can J Physiol Pharmacol. 2006 Jul;84(7):687-94. doi: 10.1139/y06-025. Can J Physiol Pharmacol. 2006. PMID: 16998532 Review. - The generation of localized calcium rises mediated by cell adhesion molecules and their role in neuronal growth cone motility.
Dunican DJ, Doherty P. Dunican DJ, et al. Mol Cell Biol Res Commun. 2000 May;3(5):255-63. doi: 10.1006/mcbr.2000.0225. Mol Cell Biol Res Commun. 2000. PMID: 10964748 Review.
Cited by
- Silencing Parkinson's risk allele Rit2 sex-specifically compromises motor function and dopamine neuron viability.
Kearney PJ, Zhang Y, Liang M, Tan Y, Kahuno E, Conklin TL, Fagan RR, Pavchinskiy RG, Shaffer SA, Yue Z, Melikian HE. Kearney PJ, et al. NPJ Parkinsons Dis. 2024 Feb 23;10(1):41. doi: 10.1038/s41531-024-00648-8. NPJ Parkinsons Dis. 2024. PMID: 38395968 Free PMC article. - Plexin B3 promotes neurite outgrowth, interacts homophilically, and interacts with Rin.
Hartwig C, Veske A, Krejcova S, Rosenberger G, Finckh U. Hartwig C, et al. BMC Neurosci. 2005 Aug 25;6:53. doi: 10.1186/1471-2202-6-53. BMC Neurosci. 2005. PMID: 16122393 Free PMC article. - The plasma membrane-associated GTPase Rin interacts with the dopamine transporter and is required for protein kinase C-regulated dopamine transporter trafficking.
Navaroli DM, Stevens ZH, Uzelac Z, Gabriel L, King MJ, Lifshitz LM, Sitte HH, Melikian HE. Navaroli DM, et al. J Neurosci. 2011 Sep 28;31(39):13758-70. doi: 10.1523/JNEUROSCI.2649-11.2011. J Neurosci. 2011. PMID: 21957239 Free PMC article. - A conserved sequence in calmodulin regulated spectrin-associated protein 1 links its interaction with spectrin and calmodulin to neurite outgrowth.
King MD, Phillips GW, Bignone PA, Hayes NV, Pinder JC, Baines AJ. King MD, et al. J Neurochem. 2014 Feb;128(3):391-402. doi: 10.1111/jnc.12462. Epub 2013 Oct 24. J Neurochem. 2014. PMID: 24117850 Free PMC article. - RIT2, a neuron-specific small guanosine triphosphatase, is expressed in retinal neuronal cells and its promoter is modulated by the POU4 transcription factors.
Zhang L, Wahlin K, Li Y, Masuda T, Yang Z, Zack DJ, Esumi N. Zhang L, et al. Mol Vis. 2013 Jun 17;19:1371-86. Print 2013. Mol Vis. 2013. PMID: 23805044 Free PMC article.
References
- Benard, V., B.P. Bohl, and G.M. Bokoch. 1999. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274:13198–13204. - PubMed
- Curtis, J., and S. Finkbeiner. 1999. Sending signals from the synapse to the nucleus: possible roles for CaMK, Ras/ERK, and SAPK pathways in the regulation of synaptic plasticity and neuronal growth. J. Neurosci. Res. 58:88–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous