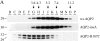Reversed polarized delivery of an aquaporin-2 mutant causes dominant nephrogenic diabetes insipidus - PubMed (original) (raw)
Reversed polarized delivery of an aquaporin-2 mutant causes dominant nephrogenic diabetes insipidus
Erik-Jan Kamsteeg et al. J Cell Biol. 2003.
Abstract
Vasopressin regulates body water conservation by redistributing aquaporin-2 (AQP2) water channels from intracellular vesicles to the apical surface of renal collecting ducts, resulting in water reabsorption from urine. Mutations in AQP2 cause autosomal nephrogenic diabetes insipidus (NDI), a disease characterized by the inability to concentrate urine. Here, we report a frame-shift mutation in AQP2 causing dominant NDI. This AQP2 mutant is a functional water channel when expressed in Xenopus oocytes. However, expressed in polarized renal cells, it is misrouted to the basolateral instead of apical plasma membrane. Additionally, this mutant forms heterotetramers with wild-type AQP2 and redirects this complex to the basolateral surface. The frame shift induces a change in the COOH terminus of AQP2, creating both a leucine- and a tyrosine-based motif, which cause the reversed sorting of AQP2. Our data reveal a novel cellular phenotype in dominant NDI and show that dominance of basolateral sorting motifs in a mutant subunit can be the molecular basis for disease.
Figures
Figure 1.
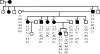
Segregation of autosomal dominant NDI within the studied family. Healthy (open symbols) and affected (filled symbols) individuals, and male (squares) and females (circles) are indicated. The 12q13 haplotype is represented using the marker order: centromere-AFM259vf9-_AQP2_-D12S131-AFMb007yg5-telomere. The lengths of simple sequence tags in nucleotides (AFM259vf9 [1:313; 2:315], D12S131 [3:156; 4:158], AFMb007yg5 [b:226; d:230; j:242]), the C or T polymorphism in exon 2, 779-780insA nucleotide insertion (i) or normal sequence (n), and the A or C polymorphism in the 3′ UTR of AQP2 are indicated.
Figure 2.
COOH termini of wt-AQP2 and AQP2-insA. The COOH-terminal amino acids (one-letter symbols) and corresponding nucleotide sequences of wild-type aquaporin-2 (wt-AQP2) and AQP2-insA are shown, starting at aa 253. The c779-780insA and c836A>C mutations, identified in the mutant AQP2 allele of the patient, are underlined and bold. An asterisk indicates a stop codon.
Figure 3.
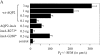
Expression of AQP2-insA in oocytes. (A) Water permeabilities. Oocytes were not injected (control), or injected with the indicated amounts of cRNA encoding wild-type AQP2 (wt-AQP2), AQP2-insA, AQP2-insA-R273* (insA-R273*), or AQP2-insA-G268* (insA-G268*). 2 d after injection, the mean water permeability (Pf ± SEM [in μm/s]; n >12) was determined in a standard swelling assay. (B) AQP2 protein expression levels. Total membranes (TM) and plasma membranes (PM) of oocytes that were used for the Pf measurements were isolated, and equivalents of four oocytes were immunoblotted. To allow comparison of AQP2 protein amounts, the membranes were incubated with AQP2 antibodies that were completely preabsorbed with a synthetic peptide raised against the last 15 amino acids of wt-AQP2 (not depicted). Complex glycosylated and nonglycosylated AQP2 proteins are indicated by c-AQP2 and AQP2, respectively. (C) Subcellular localization of AQP2-insA proteins. 2 d after injection, noninjected oocytes (control) and oocytes injected with 0.3 ng cRNA encoding the indicated AQP2 proteins were fixed and embedded in paraffin. On 5-μm sections, AQP2 was visualized using rabbit AQP2 antibodies, followed by Alexa® 594–conjugated anti–rabbit IgGs. Arrows indicate the plasma membrane.
Figure 3.
Expression of AQP2-insA in oocytes. (A) Water permeabilities. Oocytes were not injected (control), or injected with the indicated amounts of cRNA encoding wild-type AQP2 (wt-AQP2), AQP2-insA, AQP2-insA-R273* (insA-R273*), or AQP2-insA-G268* (insA-G268*). 2 d after injection, the mean water permeability (Pf ± SEM [in μm/s]; n >12) was determined in a standard swelling assay. (B) AQP2 protein expression levels. Total membranes (TM) and plasma membranes (PM) of oocytes that were used for the Pf measurements were isolated, and equivalents of four oocytes were immunoblotted. To allow comparison of AQP2 protein amounts, the membranes were incubated with AQP2 antibodies that were completely preabsorbed with a synthetic peptide raised against the last 15 amino acids of wt-AQP2 (not depicted). Complex glycosylated and nonglycosylated AQP2 proteins are indicated by c-AQP2 and AQP2, respectively. (C) Subcellular localization of AQP2-insA proteins. 2 d after injection, noninjected oocytes (control) and oocytes injected with 0.3 ng cRNA encoding the indicated AQP2 proteins were fixed and embedded in paraffin. On 5-μm sections, AQP2 was visualized using rabbit AQP2 antibodies, followed by Alexa® 594–conjugated anti–rabbit IgGs. Arrows indicate the plasma membrane.
Figure 3.
Expression of AQP2-insA in oocytes. (A) Water permeabilities. Oocytes were not injected (control), or injected with the indicated amounts of cRNA encoding wild-type AQP2 (wt-AQP2), AQP2-insA, AQP2-insA-R273* (insA-R273*), or AQP2-insA-G268* (insA-G268*). 2 d after injection, the mean water permeability (Pf ± SEM [in μm/s]; n >12) was determined in a standard swelling assay. (B) AQP2 protein expression levels. Total membranes (TM) and plasma membranes (PM) of oocytes that were used for the Pf measurements were isolated, and equivalents of four oocytes were immunoblotted. To allow comparison of AQP2 protein amounts, the membranes were incubated with AQP2 antibodies that were completely preabsorbed with a synthetic peptide raised against the last 15 amino acids of wt-AQP2 (not depicted). Complex glycosylated and nonglycosylated AQP2 proteins are indicated by c-AQP2 and AQP2, respectively. (C) Subcellular localization of AQP2-insA proteins. 2 d after injection, noninjected oocytes (control) and oocytes injected with 0.3 ng cRNA encoding the indicated AQP2 proteins were fixed and embedded in paraffin. On 5-μm sections, AQP2 was visualized using rabbit AQP2 antibodies, followed by Alexa® 594–conjugated anti–rabbit IgGs. Arrows indicate the plasma membrane.
Figure 4.
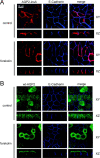
Localization of AQP2-insA in MDCK cells. MDCK cells expressing AQP2-insA (A) or wt-AQP2 (B) were grown to confluence, incubated without (control) or with forskolin, fixed, and immunostained using guinea pig AQP2-insA antibodies followed by an incubation with Alexa® 594–conjugated anti–guinea pig IgGs (red) or rabbit AQP2 antibodies, followed by Alexa® 488–conjugated anti–rabbit IgGs (green). The basolateral marker protein E-cadherin was visualized using specific rat antibodies followed by Cy5-conjugated anti–rat IgGs. Horizontal (XY) and vertical (XZ) images for AQP2-insA, wt-AQP2, and E-cadherin were obtained with a confocal laser-scanning microscope and merged (merge).
Figure 5.
Oligomerization state of AQP2-insA. (A) Homotetramerization. Membranes of MDCK cells expressing the indicated AQP2 proteins were dissolved in deoxycholate and subjected to differential velocity centrifugation. Fractions were collected from the top, of which fractions B–P were immunoblotted for AQP2. The fractions with peak intensities of the marker proteins ovalbumin (3.6 s), BSA (4.3 s), phosphorylase B (5.2 s), yeast alcohol dehydrogenase (7.4 s), and catalase (11.2 s) are indicated by arrows and by their sedimentation values. The molecular masses (in kD) of the respective AQP2 proteins are indicated on the left. (B) Heteroligomerization of AQP2-insA with wt-AQP2. Membranes of MDCK cells expressing wt-AQP2 (wt), AQP2-insA (insA), or VSV-G-tagged AQP2-insA together with wt-AQP2 (V-insA + wt) were solubilized in deoxycholate and centrifuged to remove nonsolubilized material. The supernatants were directly subjected to immunoblotting (TM, total membranes) or subjected to immunoprecipitation with affinity-purified AQP2-insA antibodies (insA IPs), followed by immunoblotting. Transferred AQP2 proteins were detected with rabbit AQP2 antibodies that recognize wt-AQP2 and AQP2-insA, or with affinity-purified rabbit AQP2 COOH-terminal antibodies (AQP2 C-term antibodies) that do not recognize AQP2-insA. Specific bands for VSV-G–tagged AQP2-insA (V-insA) and untagged AQP2 proteins (AQP2) are indicated on the right.
Figure 5.
Oligomerization state of AQP2-insA. (A) Homotetramerization. Membranes of MDCK cells expressing the indicated AQP2 proteins were dissolved in deoxycholate and subjected to differential velocity centrifugation. Fractions were collected from the top, of which fractions B–P were immunoblotted for AQP2. The fractions with peak intensities of the marker proteins ovalbumin (3.6 s), BSA (4.3 s), phosphorylase B (5.2 s), yeast alcohol dehydrogenase (7.4 s), and catalase (11.2 s) are indicated by arrows and by their sedimentation values. The molecular masses (in kD) of the respective AQP2 proteins are indicated on the left. (B) Heteroligomerization of AQP2-insA with wt-AQP2. Membranes of MDCK cells expressing wt-AQP2 (wt), AQP2-insA (insA), or VSV-G-tagged AQP2-insA together with wt-AQP2 (V-insA + wt) were solubilized in deoxycholate and centrifuged to remove nonsolubilized material. The supernatants were directly subjected to immunoblotting (TM, total membranes) or subjected to immunoprecipitation with affinity-purified AQP2-insA antibodies (insA IPs), followed by immunoblotting. Transferred AQP2 proteins were detected with rabbit AQP2 antibodies that recognize wt-AQP2 and AQP2-insA, or with affinity-purified rabbit AQP2 COOH-terminal antibodies (AQP2 C-term antibodies) that do not recognize AQP2-insA. Specific bands for VSV-G–tagged AQP2-insA (V-insA) and untagged AQP2 proteins (AQP2) are indicated on the right.
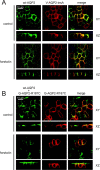
Figure 6.
Colocalization and regulation of AQP2-insA and wt-AQP2 in MDCK cells. (A) MDCK cells coexpressing wt-AQP2 and VSV-G–tagged AQP2-insA were incubated without (control) or with forskolin, fixed, and incubated with rabbit AQP2 COOH-terminal antibodies and guinea pig AQP2-insA antibodies, followed by Alexa® 488–conjugated anti–rabbit IgGs (green) and Alexa® 594–conjugated anti–guinea pig IgGs (red). Horizontal (XY) and vertical (XZ) images for wt-AQP2 and VSV-G–tagged AQP2-insA (V-AQP2-insA) were obtained with a confocal laser-scanning microscope and merged (merge). (B) MDCK cells heterologously expressing wt-AQP2 were supertransfected with a plasmid encoding GFP-tagged AQP2-R187C, and GFP-positive cells were selected using FACS® analysis. These pooled cells were grown to confluence on filter supports, treated as under A, and incubated with rabbit AQP2 COOH-terminal antibodies that recognize both wt-AQP2 and AQP2-R187C (wt-AQP2 + G-AQP2-R187C), followed by Alexa® 594–conjugated anti–rabbit IgGs (shown in green pseudocolor). The GFP signal of AQP2-R187C (G-AQP2-R187C) is given the red pseudocolor. Horizontal (XY) and vertical (XZ) images for were obtained with a confocal laser-scanning microscope and merged (merge).
Figure 7.
Subcellular localization of AQP2-insA mutants in MDCK cells. (A) MDCK cells stably expressing AQP2-S261* (wt-S261*) or AQP2-insA mutants (indicated by insA or introduced mutations; asterisks indicate stop codons) were incubated without (control) or with forskolin, fixed, and immunostained for AQP2 proteins using rabbit AQP2 and Alexa® 594–conjugated anti–rabbit antibodies. XZ images were made with a confocal laser-scanning microscope. (B) Mean values of integrated OD of surface or intracellular staining of AQP2 proteins were obtained from confocal laser-scanning microscope images. The ratio between surface and intracellular expression (ratio surface/intracellular ± SEM; n > 8) is determined from these values. Gray bars indicate this ratio from untreated cells, total bars indicate this ratio after forskolin stimulation.
Figure 7.
Subcellular localization of AQP2-insA mutants in MDCK cells. (A) MDCK cells stably expressing AQP2-S261* (wt-S261*) or AQP2-insA mutants (indicated by insA or introduced mutations; asterisks indicate stop codons) were incubated without (control) or with forskolin, fixed, and immunostained for AQP2 proteins using rabbit AQP2 and Alexa® 594–conjugated anti–rabbit antibodies. XZ images were made with a confocal laser-scanning microscope. (B) Mean values of integrated OD of surface or intracellular staining of AQP2 proteins were obtained from confocal laser-scanning microscope images. The ratio between surface and intracellular expression (ratio surface/intracellular ± SEM; n > 8) is determined from these values. Gray bars indicate this ratio from untreated cells, total bars indicate this ratio after forskolin stimulation.
Similar articles
- A novel mechanism in recessive nephrogenic diabetes insipidus: wild-type aquaporin-2 rescues the apical membrane expression of intracellularly retained AQP2-P262L.
de Mattia F, Savelkoul PJ, Bichet DG, Kamsteeg EJ, Konings IB, Marr N, Arthus MF, Lonergan M, van Os CH, van der Sluijs P, Robertson G, Deen PM. de Mattia F, et al. Hum Mol Genet. 2004 Dec 15;13(24):3045-56. doi: 10.1093/hmg/ddh339. Epub 2004 Oct 27. Hum Mol Genet. 2004. PMID: 15509592 - Three families with autosomal dominant nephrogenic diabetes insipidus caused by aquaporin-2 mutations in the C-terminus.
Kuwahara M, Iwai K, Ooeda T, Igarashi T, Ogawa E, Katsushima Y, Shinbo I, Uchida S, Terada Y, Arthus MF, Lonergan M, Fujiwara TM, Bichet DG, Marumo F, Sasaki S. Kuwahara M, et al. Am J Hum Genet. 2001 Oct;69(4):738-48. doi: 10.1086/323643. Epub 2001 Aug 30. Am J Hum Genet. 2001. PMID: 11536078 Free PMC article. - An impaired routing of wild-type aquaporin-2 after tetramerization with an aquaporin-2 mutant explains dominant nephrogenic diabetes insipidus.
Kamsteeg EJ, Wormhoudt TA, Rijss JP, van Os CH, Deen PM. Kamsteeg EJ, et al. EMBO J. 1999 May 4;18(9):2394-400. doi: 10.1093/emboj/18.9.2394. EMBO J. 1999. PMID: 10228154 Free PMC article. - Defective processing and trafficking of water channels in nephrogenic diabetes insipidus.
Kamsteeg EJ, Deen PM, van Os CH. Kamsteeg EJ, et al. Exp Nephrol. 2000 Nov-Dec;8(6):326-31. doi: 10.1159/000020686. Exp Nephrol. 2000. PMID: 11014929 Review. - Vasopressin type-2 receptor and aquaporin-2 water channel mutants in nephrogenic diabetes insipidus.
Deen PM, Knoers NV. Deen PM, et al. Am J Med Sci. 1998 Nov;316(5):300-9. doi: 10.1097/00000441-199811000-00003. Am J Med Sci. 1998. PMID: 9822112 Review.
Cited by
- Plant and Mammal Aquaporins: Same but Different.
Laloux T, Junqueira B, Maistriaux LC, Ahmed J, Jurkiewicz A, Chaumont F. Laloux T, et al. Int J Mol Sci. 2018 Feb 8;19(2):521. doi: 10.3390/ijms19020521. Int J Mol Sci. 2018. PMID: 29419811 Free PMC article. Review. - Hereditary Nephrogenic Diabetes Insipidus: Pathophysiology and Possible Treatment. An Update.
Milano S, Carmosino M, Gerbino A, Svelto M, Procino G. Milano S, et al. Int J Mol Sci. 2017 Nov 10;18(11):2385. doi: 10.3390/ijms18112385. Int J Mol Sci. 2017. PMID: 29125546 Free PMC article. Review. - Hereditary nephrogenic diabetes insipidus in Japanese patients: analysis of 78 families and report of 22 new mutations in AVPR2 and AQP2.
Sasaki S, Chiga M, Kikuchi E, Rai T, Uchida S. Sasaki S, et al. Clin Exp Nephrol. 2013 Jun;17(3):338-44. doi: 10.1007/s10157-012-0726-z. Epub 2012 Nov 14. Clin Exp Nephrol. 2013. PMID: 23150186 - Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus.
Bockenhauer D, Bichet DG. Bockenhauer D, et al. Nat Rev Nephrol. 2015 Oct;11(10):576-88. doi: 10.1038/nrneph.2015.89. Epub 2015 Jun 16. Nat Rev Nephrol. 2015. PMID: 26077742 Review. - Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia.
Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E. Deora AA, et al. Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16245-50. doi: 10.1073/pnas.0504419102. Epub 2005 Oct 31. Proc Natl Acad Sci U S A. 2005. PMID: 16260747 Free PMC article.
References
- Asai, T., M. Kuwahara, H. Kurihara, T. Sakai, Y. Terada, F. Marumo, and S. Sasaki. 2003. Pathogenesis of nephrogenic diabetes insipidus by aquaporin-2 C-terminus mutations. Kidney Int. 64:2–10. - PubMed
- Brewer, C.B., and M.G. Roth. 1995. Polarized exocytosis in MDCK cells is regulated by phosphorylation. J. Cell Sci. 108:789–796. - PubMed
- Casanova, J.E., G. Apodaca, and K.E. Mostov. 1991. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 66:65–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources