A Salmonella protein causes macrophage cell death by inducing autophagy - PubMed (original) (raw)
A Salmonella protein causes macrophage cell death by inducing autophagy
Lorraine D Hernandez et al. J Cell Biol. 2003.
Abstract
Salmonella enterica, the causative agent of food poisoning and typhoid fever, induces programmed cell death in macrophages, a process found to be dependent on a type III protein secretion system, and SipB, a protein with membrane fusion activity that is delivered into host cells by this system. When expressed in cultured cells, SipB caused the formation of and localized to unusual multimembrane structures. These structures resembled autophagosomes and contained both mitochondrial and endoplasmic reticulum markers. A mutant form of SipB devoid of membrane fusion activity localized to mitochondria, but did not induce the formation of membrane structures. Upon Salmonella infection of macrophages, SipB was found in mitochondria, which appeared swollen and devoid of christae. Salmonella-infected macrophages exhibited marked accumulation of autophagic vesicles. We propose that Salmonella, through the action of SipB, kills macrophages by disrupting mitochondria, thereby inducing autophagy and cell death.
Figures
Figure 1.
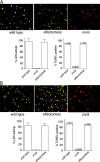
An S. typhimurium strain devoid of its SPI-1 TTSS effector proteins is able to induce programmed cell death in macrophages. BMDPM from wild-type (A) or caspase-1−/− (B) mice (Kuida et al., 1995) were infected with wild-type S. typhimurium or mutant derivative either lacking all TTSS effector proteins (effectorless) or defective for TTSS secretion by virtue a having a mutation on an essential component of this system (invG). 6 h after infection, cytotoxicity was evaluated by lactate dehydrogenase release or by terminal deoxynucleatide transferase (TUNEL) staining. Values for the lactate dehydrogenase release represent the average of at least three independent measurements. Equivalent results were obtained in several repetitions of these experiments.
Figure 2.
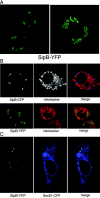
Transient expression of SipB in BMDPM from caspase-1 −/− mice results in cell death. Plasmids expressing either SipB, SipC, or SipD fused to YFP were introduced into BMDPM from caspase-1−/− mice as indicated in the Materials and methods section. Macrophages were then fixed, stained with DAPI (to visualize chromatin), and observed under a fluorescence microscope. Cells expressing SipC or SipD do not show any signs of cytotoxicity. In contrast, cells expressing SipB show clear signs of cytotoxicity and chromatin condensation. Arrows indicate the nucleus of transfected cells.
Figure 3.
Transient expression of SipB in COS-2 cells. (A) A plasmid expressing YFP-tagged SipB was transfected into COS-2 cells, and its localization was examined under a confocal microscope. (B) SipB colocalized with mitochondrial markers in transfected cells. COS-2 cells were transfected with a plasmid encoding either SipB-CFP (top) or SipB-YFP (bottom). Transfected cells were stained with MitoTracker® Red (Molecular Probes, Inc.) to visualize mitochondria and were examined by confocal microscopy. The SipB-induced structures were readily stained with mitochondrial markers (top). Mitochondria were also seen contained within the SipB-induced structures (bottom). (C) SipB-induced structures were labeled with the ER marker Sec-61. COS-2 cells were cotransfected with plasmids encoding SipB-YFP and Sec61-CFP, and transfected cells were examined by confocal microscopy.
Figure 4.
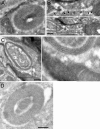
Immuno-EM of SipB-transfected COS-2 cells. COS-2 cells were transfected with a plasmid expressing SipB-YFP, and 24 h after transfection, cells were fixed and prepared for immuno-EM using an antibody specific for GFP and a gold-labeled anti–rabbit antibody. SipB was seen localized to structures made up of closely apposed membranes that were often in close proximity to mitochondria (A). Mitochondria were often seen apparently fusing to the SipB-induced membranous structure (B, see arrows in inset on top; C, inset to the right). Mitochondria were often seen contained with the SipB-induced membrane structures (D). Bars, 250 nm.
Figure 5.
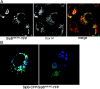
The nonfusogenic SipB428–593 mutant localizes to the mitochondria, but does not form multivesicular structures and interferes with the activity of the wild-type protein. (A) COS-2 cells were transfected with a plasmid expressing YFP-tagged SipB428–593, stained with an antibody to the mitochondrial protein CoxIV, subunit VIc (Molecular Probes, Inc.), and examined by confocal microscopy. SipB428–593-YFP localized to mitochondria and did not induce the formation of multivesicular structures seen after expression of the wild-type protein. (B) COS-2 cells were cotransfected with plasmids expressing either SipB428–593-YFP or SipB-CFP, and transfected cells were examined under a confocal microscope. Expression of SipB428–593-YFP prevented wild-type SipB-CFP from forming multivesicular structures (notice that vesicular structures are only apparent in the absence, but not in the presence, of SipB428–593-YFP; right).
Figure 6.
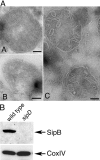
The S. typhimurium SPI-1 TTSS–secreted protein SipB localizes to the mitochondria of infected macrophages. (A) Bone marrow–derived macrophages were infected with either wild-type S. typhimurium or the TTSS translocation-defective sipD mutant strain with a multiplicity of infection of 50. 3 h after infection, macrophages were collected and processed for immuno-EM using gold-labeled antibodies. SipB could be readily detected in macrophages infected with wild-type (A and B), but not in macrophages infected with the sipD mutant strain (C). Bars, 100 nm. (B) Alternatively, mitochondria were isolated from the infected macrophages and the presence of SipB or the resident mitochondrial protein CoxIV, subunit IV, and were examined by Western immunoblot analysis using specific antibodies.
Figure 7.
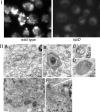
_S. typhimurium_-infected macrophages exhibited signs of autophagocytosis. Caspase-1−/− BMDPM were infected with wild-type S. typhimurium or it isogenic translocation-defective sipD mutant. 6 h after infection, macrophages were either stained with MDC to label autophagosomes (I) or processed for EM (II) as described in the legend to Fig. 3. Macrophages infected with wild-type S. typhimurium (but not those infected with the sipD mutant) exhibited a large number of vesicular structures that stained with MDC (I). Under the electron microscope (II), macrophages infected with wild-type Salmonella exhibited multivesicular structures resembling autophagosomes (A–D) Often, electron-dense material, presumably damaged organelles, were readily observed within the multivesicular structures (B–D). In contrast, macrophages infected with type III secretion-defective sipD mutant did not exhibit the large number of multivesicular structures observed in wild-type–infected cells. Bars (A and E) 500 nm; (B, C, D and F) 250 nm.
Figure 8.
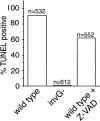
The _S. typhimurium_-induced macrophage death does not require caspase activity. BMDPM were infected with wild-type S. typhimurium or the TTSS-defective invG mutant in the presence or absence of 50 μM of the pan-caspase inhibitor Z-VAD, and cell death was measured as indicated in the legend to Fig. 1.
Similar articles
- The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1.
Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. Hersh D, et al. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2396-401. doi: 10.1073/pnas.96.5.2396. Proc Natl Acad Sci U S A. 1999. PMID: 10051653 Free PMC article. - Salmonella enterica serovar typhimurium induces cell death in bovine monocyte-derived macrophages by early sipB-dependent and delayed sipB-independent mechanisms.
Santos RL, Tsolis RM, Bäumler AJ, Smith R 3rd, Adams LG. Santos RL, et al. Infect Immun. 2001 Apr;69(4):2293-301. doi: 10.1128/IAI.69.4.2293-2301.2001. Infect Immun. 2001. PMID: 11254586 Free PMC article. - Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1.
Monack DM, Detweiler CS, Falkow S. Monack DM, et al. Cell Microbiol. 2001 Dec;3(12):825-37. doi: 10.1046/j.1462-5822.2001.00162.x. Cell Microbiol. 2001. PMID: 11736994 - Evidence and speculation: the response of Salmonella confronted by autophagy in macrophages.
Xie Z, Zhang Y, Huang X. Xie Z, et al. Future Microbiol. 2020 Sep;15:1277-1286. doi: 10.2217/fmb-2020-0125. Future Microbiol. 2020. PMID: 33026883 Review. - A smARF way to die: a novel short isoform of p19ARF is linked to autophagic cell death.
Reef S, Kimchi A. Reef S, et al. Autophagy. 2006 Oct-Dec;2(4):328-30. doi: 10.4161/auto.3107. Epub 2006 Oct 17. Autophagy. 2006. PMID: 16874094 Review.
Cited by
- Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation.
Fàbrega A, Vila J. Fàbrega A, et al. Clin Microbiol Rev. 2013 Apr;26(2):308-41. doi: 10.1128/CMR.00066-12. Clin Microbiol Rev. 2013. PMID: 23554419 Free PMC article. Review. - The scutellum of germinated wheat grains undergoes programmed cell death: identification of an acidic nuclease involved in nucleus dismantling.
Domínguez F, Moreno J, Cejudo FJ. Domínguez F, et al. J Exp Bot. 2012 Sep;63(15):5475-85. doi: 10.1093/jxb/ers199. Epub 2012 Aug 9. J Exp Bot. 2012. PMID: 22888125 Free PMC article. - Exploitation of eukaryotic subcellular targeting mechanisms by bacterial effectors.
Hicks SW, Galán JE. Hicks SW, et al. Nat Rev Microbiol. 2013 May;11(5):316-26. doi: 10.1038/nrmicro3009. Nat Rev Microbiol. 2013. PMID: 23588250 Free PMC article. Review. - Salmonella enterica serovar Typhimurium remodels mitochondrial dynamics of macrophages via the T3SS effector SipA to promote intracellular proliferation.
Liu X, Liu Y, Zhao X, Li X, Yao T, Liu R, Wang Q, Wang Q, Li D, Chen X, Liu B, Feng L. Liu X, et al. Gut Microbes. 2024 Jan-Dec;16(1):2316932. doi: 10.1080/19490976.2024.2316932. Epub 2024 Feb 14. Gut Microbes. 2024. PMID: 38356294 Free PMC article. - Bacterial Manipulation of Autophagic Responses in Infection and Inflammation.
Jiao Y, Sun J. Jiao Y, et al. Front Immunol. 2019 Dec 3;10:2821. doi: 10.3389/fimmu.2019.02821. eCollection 2019. Front Immunol. 2019. PMID: 31849988 Free PMC article. Review.
References
- Biederbick, A., H. Kern, and H. Elsasser. 1995. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur. J. Cell Biol. 66:3–14. - PubMed
- Boise, L., and C. Collins. 2001. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 9:64–67. - PubMed
- Brennan, M.A., and B.T. Cookson. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38:31–40. - PubMed
- Bursch, W., A. Ellinger, C. Gerner, U. Frohwein, and R. Schulte-Hermann. 2000. Programmed cell death (PCD). Apoptosis, autophagic PCD, or others? Ann. NY Acad. Sci. 926:1–12. - PubMed
- Chen, L.M., K. Kaniga, and J.E. Galán. 1996. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 21:1101–1115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources