Migration and differentiation of neural precursor cells can be directed by microglia - PubMed (original) (raw)
Migration and differentiation of neural precursor cells can be directed by microglia
Johan Aarum et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Recent reports have supported the existence of neural stem cells in the adult mammalian CNS. Important features of such cells are self-renewal and multipotency, i.e., they can give rise to neurons, astrocytes, and oligodendrocytes and thus in principle replace lost cells in the CNS. Observations in several animal models of CNS diseases have shown that by unknown mechanisms endogenous as well as exogenous precursor cells preferentially migrate to damaged areas. Microglia are immunoreactive cells of nonneural lineage resident in the CNS. After injury to the CNS, microglia are rapidly activated and found concentrated at the sites of injury. In the present article we show, in two different assays, that soluble factors released from mouse microglial cells direct the migration of neural CNS precursor cells. We also provide evidence that microglia have the capacity to influence the differentiation of both adult and embryonic neural precursor cells toward a neuronal phenotype. Given that an invariant feature of pathological processes in CNS is the activation of microglia, these results indicate an important and unique role for microglia in directing the replacement of damaged or lost cells in the CNS.
Figures
Fig. 2.
Migration of precursor cells from EGF-treated aggregates in Matrigel. (A) Precursor cells migrate radially from EGF-treated aggregate when embedded in Matrigel. (B) Cells migrated both as single cells frequently attached to long processes (arrows) and tightly packed together. (C) No cells migrated from aggregates not pretreated with EGF. (D_–_G) Pseudocolored immunofluorescence micrographs of the same EGF-treated aggregate 6 days after embedding in Matrigel. Aggregates were immunostained for βIII (neurons, red) (D) and GFAP (astrocytes, green) (E) and counterstained with DAPI (nuclei, blue) (F). (G) Overlay picture of D_–_F. (Scale bars, 50 μm.) (H) Schematic drawing showing the experimental principle and the overlaid quadrants used to quantify precursor migration from the embedded aggregate in the centre. The proximal (p) and the distal (d) quadrants relative to the conditioned media.
Fig. 1.
EGF-treated mixed CNS cell aggregates contain neural precursor cells. (A_–_C) Bright-field micrographs displaying EGF-treated aggregates, with an outer ring area of nestin-positive precursor cells (A). The outer ring of cells lack the expression of the neuronal marker βIII (B) and the astrocytic marker GFAP (C). Consecutive sections from the same aggregate are shown. (Scale bar, 50 μm.)
Fig. 3.
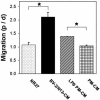
Directed migration of precursor cells in Matrigel toward microglial-conditioned media. Ratio between number of cells in the proximal quadrant (p) and distal quadrant (d) as described in Fig. 2_H_. Data are the mean from 12 aggregates per treatment ± SEM. *, P < 0.05 by two-tailed t test.
Fig. 4.
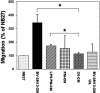
Transwell migration of precursor cells in modified Boyden chambers. Percentage of cells per microscopic field migrating toward conditioned media, relative to the spontaneous migration toward NB27 (100%). BV-2/N13-CM U/L represent the case where conditioned media were placed in both the upper (U) and lower (L) chamber. Values are given as mean ± SEM of two to four independent experiments. *, P < 0.05 by two-tailed t test.
Fig. 5.
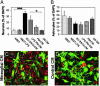
Increased proportion of neurons from embryonic neural precursor cells in microglial-conditioned media. (A and B) Percentages of neurons (βIII+ cells) (A) and astrocytes (GFAP+ cells) (B) of DAPI+ cells, after 7 days in conditioned media from microglial cells or controls. Data are expressed as mean ± SEM of positively stained cells per microscopic field and were counted blind from two to four independent experiments. ***, P < 0.001; *, P < 0.05 (by two-tailed t test). (C and D). Fluorescence overlay micrographs showing the morphology of neurons (red) and astrocytes (green) in BV-2/N13 (C) or C6 (D) conditioned media. Cells were counterstained with DAPI (blue). (Scale bar, 50 μm.)
Fig. 6.
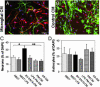
Differentiation of adult neural precursor cells in microglial-conditioned media. (A and B) Fluorescence overlay micrographs of adult neural precursor cells cultured for 7 days in BV-2/N13-CM (A) or NB27 (B). Neurons were identified with anti-βIII (red) and astrocytes with anti-GFAP (green). Cells were counterstained with DAPI (blue). (Scale bar, 50 μm.) (C and D) Proportions of neurons (C) and astrocytes (D) of DAPI+ cells in cultures of adult neural precursor cells treated with conditioned media from microglia or control cells. Bars represent mean ± SEM from two to four independent experiments. **, P < 0.01; *, P < 0.05 (by two-tailed t test).
Similar articles
- Microglial precursors derived from mouse embryonic stem cells.
Napoli I, Kierdorf K, Neumann H. Napoli I, et al. Glia. 2009 Nov 15;57(15):1660-71. doi: 10.1002/glia.20878. Glia. 2009. PMID: 19455585 - Endogenous microglia regulate development of embryonic cortical precursor cells.
Antony JM, Paquin A, Nutt SL, Kaplan DR, Miller FD. Antony JM, et al. J Neurosci Res. 2011 Mar;89(3):286-98. doi: 10.1002/jnr.22533. Epub 2011 Jan 6. J Neurosci Res. 2011. PMID: 21259316 - Neural stem cells in the subventricular zone are a source of astrocytes and oligodendrocytes, but not microglia.
Levison SW, Druckman SK, Young GM, Basu A. Levison SW, et al. Dev Neurosci. 2003 Mar-Aug;25(2-4):184-96. doi: 10.1159/000072267. Dev Neurosci. 2003. PMID: 12966216 - Neural precursor cells: applications for the study and repair of the central nervous system.
Fisher LJ. Fisher LJ. Neurobiol Dis. 1997;4(1):1-22. doi: 10.1006/nbdi.1997.0137. Neurobiol Dis. 1997. PMID: 9258907 Review. - Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity.
Nishiyama A, Komitova M, Suzuki R, Zhu X. Nishiyama A, et al. Nat Rev Neurosci. 2009 Jan;10(1):9-22. doi: 10.1038/nrn2495. Nat Rev Neurosci. 2009. PMID: 19096367 Review.
Cited by
- The intricate interplay between microglia and adult neurogenesis in Alzheimer's disease.
Früholz I, Meyer-Luehmann M. Früholz I, et al. Front Cell Neurosci. 2024 Sep 18;18:1456253. doi: 10.3389/fncel.2024.1456253. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39360265 Free PMC article. Review. - Microglia during development and aging.
Harry GJ. Harry GJ. Pharmacol Ther. 2013 Sep;139(3):313-26. doi: 10.1016/j.pharmthera.2013.04.013. Epub 2013 Apr 30. Pharmacol Ther. 2013. PMID: 23644076 Free PMC article. Review. - Minocycline treatment ameliorates interferon-alpha- induced neurogenic defects and depression-like behaviors in mice.
Zheng LS, Kaneko N, Sawamoto K. Zheng LS, et al. Front Cell Neurosci. 2015 Jan 28;9:5. doi: 10.3389/fncel.2015.00005. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25674053 Free PMC article. - The Participation of Microglia in Neurogenesis: A Review.
Pérez-Rodríguez DR, Blanco-Luquin I, Mendioroz M. Pérez-Rodríguez DR, et al. Brain Sci. 2021 May 18;11(5):658. doi: 10.3390/brainsci11050658. Brain Sci. 2021. PMID: 34070012 Free PMC article. Review. - Inflammaging and Brain Aging.
Jurcau MC, Jurcau A, Cristian A, Hogea VO, Diaconu RG, Nunkoo VS. Jurcau MC, et al. Int J Mol Sci. 2024 Sep 30;25(19):10535. doi: 10.3390/ijms251910535. Int J Mol Sci. 2024. PMID: 39408862 Free PMC article. Review.
References
- Alvarez-Buylla, A., Seri, B. & Doetsch, F. (2002) Brain Res. Bull. 57, 751-758. - PubMed
- Nunes, M. C., Roy, N. S., Keyoung, H. M., Goodman, R. R., McKhann, G., II, Jiang, L., Kang, J., Nedergaard, M. & Goldman, S. A. (2003) Nat. Med. 9, 439-447. - PubMed
- Palmer, T. D., Ray, J. & Gage, F. H. (1995) Mol. Cell. Neurosci. 6, 474-486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases