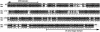Use of Sindbis virus-mediated RNA interference to demonstrate a conserved role of Broad-Complex in insect metamorphosis - PubMed (original) (raw)
Use of Sindbis virus-mediated RNA interference to demonstrate a conserved role of Broad-Complex in insect metamorphosis
Mirka Uhlirova et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The transcription factor Broad-Complex (BR-C) is required for differentiation of adult structures as well as for the programmed death of obsolete larval organs during metamorphosis of the fruit fly Drosophila melanogaster. Whether BR-C has a similar role in other holometabolous insects could not be proven without a loss-of-function genetic test, performed in a non-drosophilid species. Here we use a recombinant Sindbis virus as a tool to silence BR-C expression in the silkmoth Bombyx mori. The virus expressing a BR-C antisense RNA fragment reduced endogenous BR-C mRNA levels in infected tissues (adult wing and leg primordia) via RNA interference (RNAi). The RNAi knock-down of BR-C resulted in the failure of animals to complete the larval-pupal transition or in later morphogenetic defects, including differentiation of adult compound eyes, legs, and wings from their larval progenitors. BR-C RNAi also perturbed the programmed cell death of larval silk glands. These developmental defects correspond to loss-of-function phenotypes of BR-C Drosophila mutants in both the morphogenetic and degenerative aspects, suggesting that the critical role of BR-C in metamorphosis is evolutionarily conserved. We also demonstrate that the Sindbis virus is a useful vehicle for silencing of developmental genes in new insect models.
Figures
Fig. 1.
Amino acid identity between the BR-C Z4 isoform of M. sexta (top, AF0326761) and the protein sequence deduced from the B. mori BR-C cDNA fragment is 88%. The highly conserved C-terminal part of the BTB domain and the N-terminal portion of the Z4 zinc finger are indicated by arrows; between them is a variable part of the core, not conserved in Drosophila. The dashed line indicates which portion of the Bombyx cDNA was used for cloning into TE 3′2J-based SINV vectors (Fig. 2) and as a probe for hybridizations in Fig. 7.
Fig. 7.
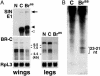
SINV mediates specific BR-C silencing via RNAi. (A) Northern blot hybridization shows that infection with TE 3′2J-Bras reduced the levels of BR-C mRNAs. Total RNA was isolated from wing discs (Left) and legs (Right) of fifth-instar larvae, either uninfected or 6 days after virus injection. The _Bombyx BR-C_-specific probe detected three transcripts. To show equal RNA loading, the membranes were rehybridized with a probe for a constitutive ribosomal protein RpL3. Hybridization with a probe for the Sindbis E1 structural protein (for wing discs) showed the full-length viral genomic RNA (arrow) and the first subgenomic viral RNA before (black arrowhead) and after (open arrowhead) deletion, which probably removed RNA at the 3′ end. N, uninfected larvae; C, control TE 3′2J virus; Bras, TE3′2J-Bras virus. (B) Hybridization of small RNAs, isolated from mixed tissues of fifth-instar larvae 6 days after infection with TE 3′2J-Bras (right lane) but not the control TE 3′2J virus (left lane) shows _BR-C_-specific siRNAs of the indicated size range.
Fig. 2.
Design and replication of recombinant SINV. Recombinant TE 3′2J-based viruses contain a full Sindbis viral genome (13.8 kb in length), represented by single-stranded positive sense RNA with a second subgenomic promoter added at the 3′ end (17). Parts of the construct are not to scale in this scheme. (A) The 705-bp BR-C cDNA fragment was cloned in antisense orientation downstream of the second subgenomic promoter, thus generating TE 3′2J-Bras (top). On infection, a negative sense RNA copy of the genome (–RNA) is made by viral RNA-dependent RNA polymerase from a signaling sequence (*) in the 3′ noncoding region of the viral genome. The–RNA serves as a template for first and second subgenomic RNAs produced from two internal promoters and for new full-length positive sense RNA. (B) The chimeric TE 3′2J-EGFP-Bras virus contains a fusion of the EGFP coding region and the antisense Bombyx BR-C cDNA. The TE 3′2J-EGFP and TE 3′2J (bottom) viruses without the BR-C insert were used for controls.
Fig. 3.
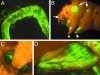
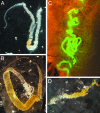
Various tissues of the silkmoth are SINV targets. The TE 3′2J-EGFP virus was injected into fourth-instar larvae. Expression of EGFP serves as a marker of successful viral replication and shows the temporal spreading of the infection. (A) The middle part of the silk gland in a fifth-instar larva. (B) Strong EGFP signals in wing discs (arrows), imaginal leg primordia (arrowhead), and eye primordia in the head (arrow) are visible through the cuticle of a fifth-instar larva. Asterisk shows a leg apparently not infected with the virus. (C and D) Infected animals survive metamorphosis and show the viral EGFP expression in adult organs such as the compound eye (C) and forewing (D), both derived from infected imaginal progenitors.
Fig. 4.
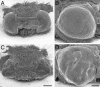
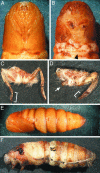
Differentiation of compound eyes is disrupted by BR-C RNAi. Shown are scanning electron micrographs of eyes from adults infected as day 0 fifth-instar larvae with the control TE 3′2J virus (A and B) and with TE 3′2J-Bras (C and D). Compared to controls, animals carrying TE 3′2J-Bras show cone-shaped eyes with invaginations and folds. The bars correspond to 500 μm in A and C and to 200 μm in B and D.
Fig. 5.
BR-C function is required for pupation, elongation, and differentiation of adult legs and wings. Most larvae infected on day 0 of the fifth instar undergo the larval–pupal transition. Compared to a control pupa carrying the TE 3′2J virus (A), a pupa infected with TE 3′2J-Bras displays short forewings and short malformed legs (B). The arrowhead in B indicates where the wings should extend and meet normally. A mesothoracic leg of a control TE 3′2J infected adult (C) shows the normal number and size of segments, whereas infection with TE 3′2J-Bras leads to deletions of segments and overall leg malformation (D). Brackets show the normal (C) and shortened (D) tarsi; arrow in D points to an undeveloped mesothoracic leg. (E and F) Although a majority of animals injected as day 2 fourth-instar larvae with control viruses form normal pupae (E), most of those infected with TE 3′2J-Bras die when trying to ecdyse (F).
Fig. 6.
BR-C plays a role in the programmed cell death of larval silk glands. Animals infected as day 2 fourth-instar larvae were dissected 12 h after pupation. Silk glands found in control TE 3′2J-infected pupae displayed late phase of histolysis in the anterior and middle parts (A). In contrast, a middle gland dissected from a TE 3′2J-Bras-infected animal showed no signs of degeneration (B). In a day 1 pupa infected with TE 3′2J-EGFP-Bras, only one gland from the pair failed to degenerate (C); shown is the posterior part where the EGFP fluorescence indicates viral infection. The middle parts of silk glands were still visible in a day 3 pupa infected with TE 3′2J-Bras (D) but not in control pupae (not shown).
Similar articles
- Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects.
Ureña E, Manjón C, Franch-Marro X, Martín D. Ureña E, et al. Proc Natl Acad Sci U S A. 2014 May 13;111(19):7024-9. doi: 10.1073/pnas.1401478111. Epub 2014 Apr 28. Proc Natl Acad Sci U S A. 2014. PMID: 24778249 Free PMC article. - Antagonistic role of the BTB-zinc finger transcription factors Chinmo and Broad-Complex in the juvenile/pupal transition and in growth control.
Chafino S, Giannios P, Casanova J, Martín D, Franch-Marro X. Chafino S, et al. Elife. 2023 Apr 28;12:e84648. doi: 10.7554/eLife.84648. Elife. 2023. PMID: 37114765 Free PMC article. - Krüppel Homolog 1 Inhibits Insect Metamorphosis via Direct Transcriptional Repression of Broad-Complex, a Pupal Specifier Gene.
Kayukawa T, Nagamine K, Ito Y, Nishita Y, Ishikawa Y, Shinoda T. Kayukawa T, et al. J Biol Chem. 2016 Jan 22;291(4):1751-1762. doi: 10.1074/jbc.M115.686121. Epub 2015 Oct 30. J Biol Chem. 2016. PMID: 26518872 Free PMC article. - Transcriptional regulation of cuticular genes during insect metamorphosis.
Ali MS, Takaki K. Ali MS, et al. Front Biosci (Landmark Ed). 2020 Jan 1;25(1):106-117. doi: 10.2741/4796. Front Biosci (Landmark Ed). 2020. PMID: 31585879 Review. - Cell death during complete metamorphosis.
Tettamanti G, Casartelli M. Tettamanti G, et al. Philos Trans R Soc Lond B Biol Sci. 2019 Oct 14;374(1783):20190065. doi: 10.1098/rstb.2019.0065. Epub 2019 Aug 26. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31438818 Free PMC article. Review.
Cited by
- Broad-complex Z3 contributes to the ecdysone-mediated transcriptional regulation of the vitellogenin gene in Bombus lantschouensis.
Zhen C, Yang H, Luo S, Huang J, Wu J. Zhen C, et al. PLoS One. 2018 Nov 15;13(11):e0207275. doi: 10.1371/journal.pone.0207275. eCollection 2018. PLoS One. 2018. PMID: 30440013 Free PMC article. - Comparative transcriptomic analysis of deep- and shallow-water barnacle species (Cirripedia, Poecilasmatidae) provides insights into deep-sea adaptation of sessile crustaceans.
Gan Z, Yuan J, Liu X, Dong D, Li F, Li X. Gan Z, et al. BMC Genomics. 2020 Mar 17;21(1):240. doi: 10.1186/s12864-020-6642-9. BMC Genomics. 2020. PMID: 32183697 Free PMC article. - Functional role of aspartic proteinase cathepsin D in insect metamorphosis.
Gui ZZ, Lee KS, Kim BY, Choi YS, Wei YD, Choo YM, Kang PD, Yoon HJ, Kim I, Je YH, Seo SJ, Lee SM, Guo X, Sohn HD, Jin BR. Gui ZZ, et al. BMC Dev Biol. 2006 Oct 25;6:49. doi: 10.1186/1471-213X-6-49. BMC Dev Biol. 2006. PMID: 17062167 Free PMC article. - Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster.
Minakuchi C, Zhou X, Riddiford LM. Minakuchi C, et al. Mech Dev. 2008 Jan-Feb;125(1-2):91-105. doi: 10.1016/j.mod.2007.10.002. Epub 2007 Oct 11. Mech Dev. 2008. PMID: 18036785 Free PMC article. - Regulatory mechanisms underlying the specification of the pupal-homologous stage in a hemimetabolous insect.
Ishimaru Y, Tomonari S, Watanabe T, Noji S, Mito T. Ishimaru Y, et al. Philos Trans R Soc Lond B Biol Sci. 2019 Oct 14;374(1783):20190225. doi: 10.1098/rstb.2019.0225. Epub 2019 Aug 26. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31438810 Free PMC article.
References
- Fristrom, D. & Fristrom, J. W. (1993) in The Development of Drosophila melanogaster, eds. Bate, M. & Martinez Adrias, A. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 843–897.
- Jiang, C., Baehrecke, E. H. & Thummel, C. S. (1997) Dev. Biol. 186, S13.
- Riddiford, L. M. (1996) Arch. Insect Biochem. Physiol. 32, 271–286. - PubMed
- Bayer, C. A., Holley, B. & Fristrom, J. W. (1996) Dev. Biol. 177, 1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI46435/AI/NIAID NIH HHS/United States
- R01 AI046435-02/AI/NIAID NIH HHS/United States
- R03TW01209-01/TW/FIC NIH HHS/United States
- AI46753/AI/NIAID NIH HHS/United States
- R01 AI046753/AI/NIAID NIH HHS/United States
- R01 AI046435/AI/NIAID NIH HHS/United States
- R01 AI046435-03/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources